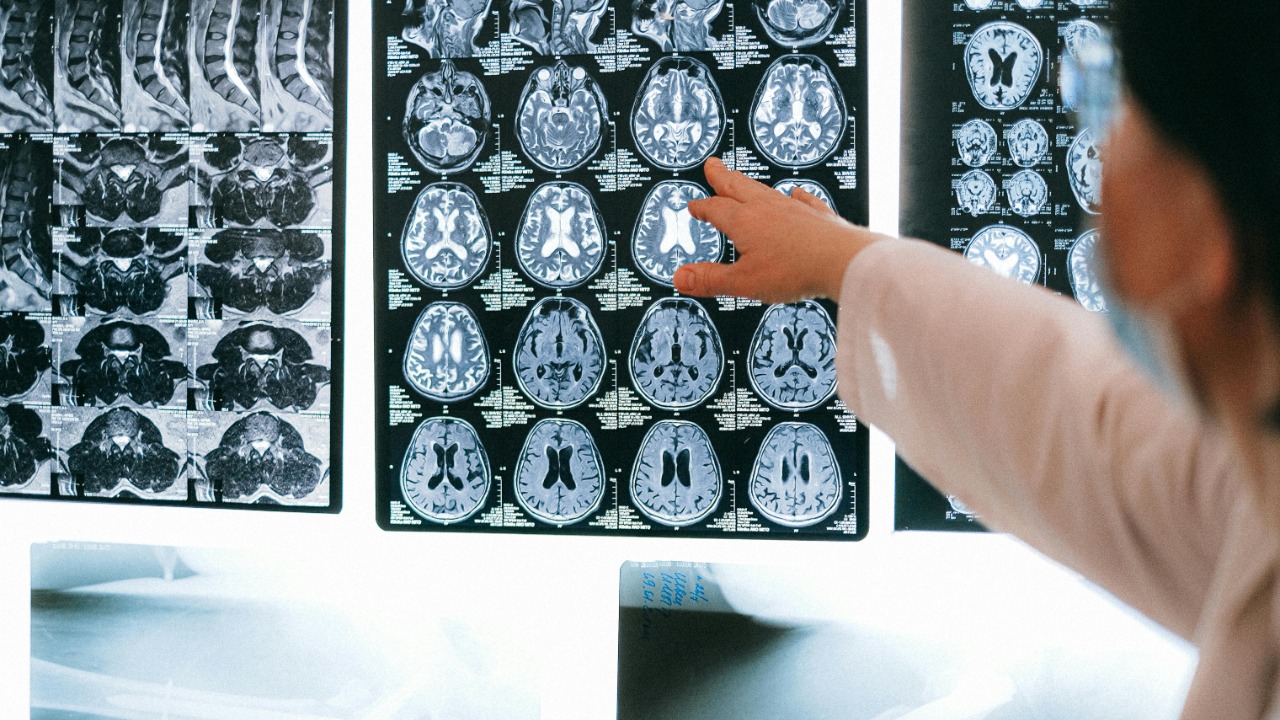
For years, scientists have known that the aging brain tends to shrink, but they have struggled to explain why some people suddenly start forgetting names, appointments, or why they walked into a room. A new mega-analysis of roughly 10,000 brain scans and memory tests now points to a clearer answer: memory decline in healthy aging is not driven by a single failing spot in the brain, but by gradual, widespread changes that eventually reach a tipping point. Instead of a slow, even slide, the data show that memory can hold steady for decades and then accelerate downward once enough of the brain’s memory network has thinned.
That shift in understanding matters far beyond the lab. It suggests that the real story behind midlife forgetfulness and later-life memory loss is about the health of entire brain networks, not just the fate of one region or one gene. It also reframes what “normal aging” looks like, and why some people stay sharp into their eighties while others feel their recall falter much earlier.
The mega-analysis that changed the aging memory story
The new work is built on an unusually large collaboration that pooled structural brain scans and memory scores from thousands of people across the adult lifespan. Instead of focusing on a single clinic or country, researchers combined data from multiple cohorts into one coordinated analysis, allowing them to track how brain volume and memory performance move together from early adulthood into old age. That scale is what makes the roughly 10,000 scans so powerful: it captures subtle patterns that smaller studies would miss, including the point at which normal variation hardens into a consistent trend.
Jan reports that the project was designed to test a deceptively simple question: does memory decline map neatly onto shrinkage in one key structure, or does it reflect a broader reorganization of the aging brain. The answer, drawn from this international dataset, is that memory performance tracks a gradual pattern of volume loss across multiple regions, with the hippocampus showing the largest effects and other areas still contributing in meaningful ways, a pattern that results from Jan describe as incompatible with a single-cause explanation.
Not one “memory center,” but a vulnerable network
For decades, the hippocampus has been cast as the brain’s memory hub, and it does emerge as the most affected region in this work. Yet the new findings show that memory scores are also tied to changes in several connected areas, including cortical regions that support attention, planning, and the ability to organize information. In other words, the scans reveal that memory decline in healthy aging reflects large-scale, network-level shifts rather than damage to one isolated “memory center,” a conclusion underscored by a massive international dataset highlighted by Jan.
That network view is echoed in commentary from Alvaro Pascual, Leone, who is identified as a Senior Scientist and emphasizes that the study links memory performance to coordinated changes across several brain regions, not just the hippocampus. In his description of the work, the pooled scans and tests show that multiple cortical and subcortical regions demonstrate significant relationships with memory, reinforcing the idea that what people experience as “forgetfulness” is the output of a stressed network rather than a single failing part.
Why memory can suddenly speed up its decline
One of the most striking patterns in the data is that memory loss does not always creep in slowly and evenly. Instead, many people show relatively stable performance for years, followed by a noticeable acceleration in their sixties or seventies. Reporting from Jan on this work notes that memory loss in later life does not always follow a smooth trajectory and can show a sudden change in slope, a pattern that appears consistently across the large sample of adults studied for Memory decline.
Researchers describe this as a tipping-point effect: as multiple regions in the memory network gradually lose volume, the system can compensate for a long time, then reach a threshold where small additional losses translate into much larger drops in day-to-day recall. Jan notes that the team observed a gradual pattern across regions, with the hippocampus showing the largest effects and smaller but still significant changes in other areas, and that this pattern helps explain why memory loss can suddenly speed up with age, as detailed by Researchers analyzing the same dataset.
Healthy aging, Alzheimer’s, and what this study is not
It is important to note that this mega-analysis focused on healthy adults, not people diagnosed with Alzheimer’s disease or other dementias. The researchers were explicit that the memory changes they tracked are part of typical aging, even if they can feel alarming when they first appear. Jan highlights that the consistency of the pattern across thousands of participants supports the idea that normal memory decline reflects large-scale and network-level changes, not just a single region or gene associated with Alzheimer’s disease, a distinction that is emphasized in a groundbreaking summary shared by Jan.
That does not mean the findings are irrelevant to dementia. By clarifying how memory typically changes with age, the study gives clinicians a more precise baseline for spotting when something is off that pattern. The work also suggests that interventions aimed at preserving memory should not target only one structure or one molecular pathway, but instead support the resilience of entire brain networks. Jan’s description of the project as a new mega-analysis that reveals relationships across multiple regions, rather than isolated pathology, underscores why the 10,000 scans are being treated as a reference point for future Alzheimer’s research.
Sex differences, lifestyle stakes, and what you can do
The pooled dataset also allowed researchers to look at how brain aging differs between men and women. A large study from the University of Oslo, folded into the broader collaboration, found that men’s brains shrink faster than women’s during healthy aging. Jan notes that Researchers at the University of Oslo reported steeper volume loss in male participants, even when memory performance remained similar, suggesting that women may have more structural reserve in some regions of the memory network.
Those sex differences do not mean that men are destined for worse memory, or that women are automatically protected. Instead, they highlight how biology and life experience interact with the same underlying network-level changes. A related report from Jan on the same University of Oslo work stresses that understanding these patterns could help tailor strategies for preventing cognitive decline and dementia, since the same structural changes may have different functional consequences in different people. By showing that men’s brains shrink faster while women may show different trajectories in specific regions, the Researchers argue that lifestyle interventions, medical monitoring, and even clinical trial designs should account for sex-specific brain aging rather than assuming a one-size-fits-all pattern.
More from Morning Overview