Post-traumatic stress disorder is usually framed as a psychological wound, but a growing body of evidence suggests it can also speed up how quickly the brain itself appears to age. Instead of simply changing mood or memory, PTSD seems to leave a biological imprint that looks a lot like accelerated wear and tear on neural tissue. I want to unpack what that means, how scientists are measuring it, and why it matters for anyone living with trauma.
Researchers are now converging on a striking idea: chronic traumatic stress does not just haunt the mind, it may push the brain and body along an older trajectory years ahead of schedule. From 911 first responders to military veterans and civilians, the pattern that is emerging is that PTSD is linked to changes in brain structure, cellular aging markers, and dementia risk that resemble an early arrival of late-life disease.
PTSD reshapes the brain’s core circuitry
To understand why PTSD might be tied to faster brain aging, I first need to look at how the condition alters neural circuits in the first place. PTSD is more than an emotional struggle, it physically rewires key regions that handle fear, memory, and decision making, including the amygdala, prefrontal cortex, and hippocampus. Over time, this pattern of heightened threat detection and impaired regulation can leave the brain locked into a state of chronic alarm, which is exactly the kind of environment that tends to accelerate biological aging in other organs as well, as shown in work on how PTSD is more than an emotional struggle.
When the brain is repeatedly pushed into fight-or-flight mode, stress hormones surge, sleep is disrupted, and the usual cycles of repair and consolidation are interrupted. I see this as the starting point for a cascade that can gradually erode gray matter volume, weaken connections between cortical and subcortical structures, and make the brain more vulnerable to later-life conditions like dementia. The same neural hubs that are reshaped by trauma are also the ones that typically thin and shrink with age, which is why scientists are now asking whether PTSD effectively presses fast-forward on that process.
What “accelerated brain age” actually means
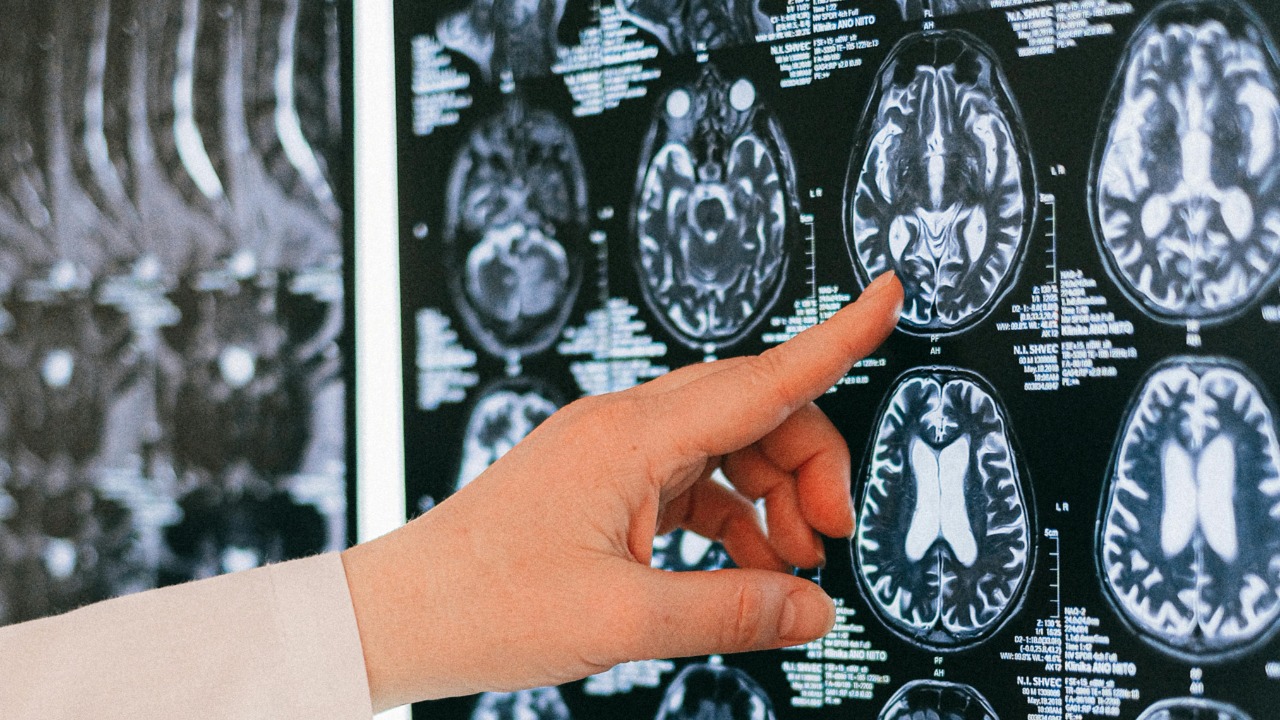
The phrase “accelerated brain age” sounds dramatic, but in research it has a specific meaning that helps me separate hype from substance. Scientists use structural and functional MRI scans to estimate how old a brain looks based on patterns of cortical thickness, white matter integrity, and network connectivity, then compare that estimate to a person’s actual age. When the scan-based prediction comes out older than the calendar age, the gap is interpreted as a sign that the brain has undergone changes usually seen later in life, and recent MRI work on brain age has applied this approach directly to PTSD.
In people with chronic PTSD, these MRI-based models often show a larger brain age gap, suggesting that trauma-related changes are not just different, they are directionally similar to aging. I find that distinction important, because it implies that PTSD is not creating an entirely new kind of brain, it is nudging the organ along a path that healthy brains eventually follow, just more quickly. That framing helps explain why PTSD is increasingly being linked to age-related outcomes like cognitive decline and dementia, and why researchers are now treating brain age as a measurable biomarker rather than a metaphor.
9/11 responders and the Mount Sinai brain aging signal
The most vivid example of this pattern comes from 911 first responders who spent months amid the toxic dust and psychological shock of the World Trade Center site. Researchers from the Icahn School of Medicine at Mount Sinai have reported that responders with PTSD show signs of older-appearing brains compared with peers of the same age who did not develop the disorder, suggesting that the combination of extreme trauma and ongoing symptoms may be linked to accelerated neurobiological aging in this group of Researchers from the Icahn School of Medicine at Mount Sinai.
In parallel, coverage of the same cohort has emphasized that post-traumatic stress disorder or PTSD may be linked to accelerated brain aging among 911 first responders, highlighting how a single disaster can leave a long tail of neurological risk that stretches decades beyond the initial event. I see this as a crucial warning that the costs of large-scale trauma are not confined to the immediate aftermath, and that monitoring brain health in such populations is not a luxury but a necessity, as underscored in a PTSD possibly linked to accelerated brain aging in 9/11 first responders report.
From oxidative stress to structural wear and tear
To move from correlation to plausible mechanism, I have to look inside the cells themselves. Chronic PTSD constitutes a form of persistent life stress that can drive oxidative stress, or OXS, a biochemical state in which reactive oxygen species outpace the brain’s antioxidant defenses and begin to damage lipids, proteins, and DNA. Reviews of traumatic stress have identified OXS as a key pathway by which PTSD may promote neurodegeneration, with postmortem studies implicating oxidative damage, hypothalamic pituitary adrenal axis activation, and sleep disturbance in the gradual breakdown of cortical and subcortical structures in people with PTSD.
When I connect those dots, a picture emerges of a brain that is being slowly eroded by the same forces that drive normal aging, only at a higher intensity and over a longer span of adult life. Oxidative stress does not just injure neurons in isolation, it also disrupts glial support, blood brain barrier integrity, and synaptic pruning, all of which are central to how the brain maintains itself over time. In that context, the structural differences seen on MRI in PTSD, such as reduced hippocampal volume or altered connectivity between frontal and limbic regions, look less like isolated scars and more like the cumulative footprint of years of stress-related wear and tear.
Cellular aging: epigenetics and DNA methylation clocks
Brain scans tell one part of the story, but I find the cellular data just as compelling. Traumatic stress-related accelerated aging has been documented at the molecular level, where researchers track changes in DNA methylation patterns that function as biological clocks. These epigenetic signatures, which normally shift gradually with age, appear to tick faster in people exposed to severe trauma, suggesting that the stress is leaving a lasting mark on gene regulation across multiple tissues, as summarized in work on Traumatic Stress and Accelerated Cellular Aging: From Epigenetics.
One influential line of research has focused on accelerated DNA methylation age and its associations with PTSD, finding that accumulating evidence suggests that post traumatic stress disorder may accelerate cellular aging and alter the trajectory of age-related disease risk. In these studies, individuals with PTSD often show a higher epigenetic age than their chronological age would predict, which I interpret as a molecular echo of the same acceleration seen in brain imaging, as detailed in analyses of Accelerated DNA Methylation Age: Associations with PTSD.
Dementia risk and the long shadow of trauma
If PTSD is nudging both brain structure and cellular clocks toward an older profile, the next question I have to ask is whether that translates into more dementia. Evidence from male veterans indicates that post-traumatic stress disorder is associated with an increased risk of dementia, with hazard ratios that remain elevated even after adjusting for other health factors. In one large cohort, PTSD was linked to a significantly higher incidence of cognitive decline, reinforcing the idea that trauma is not just a mental health issue but a long-term neurological risk factor, as shown in work where the INTRODUCTION, Post, PTSD connection is quantified.
For me, this is where the concept of accelerated brain aging becomes more than an academic curiosity. If PTSD increases the likelihood that someone will develop dementia earlier or more severely than they otherwise would, then early detection and treatment of trauma symptoms become a form of dementia prevention. It also means that clinicians should be alert to subtle cognitive changes in older adults with a history of PTSD, rather than attributing memory lapses or executive difficulties solely to normal aging, because the underlying biology may be primed for faster decline.
Why 9/11 responders became a pivotal case study
The 911 responder cohort has become a kind of natural experiment in how extreme trauma and environmental exposure intersect with brain aging. Many of these individuals were relatively young adults at the time of the attacks, which allows researchers to track how PTSD symptoms, toxic exposures, and lifestyle factors interact over decades. Reports on this group emphasize that PTSD may accelerate brain aging in 9/11 responders, and that those with more severe symptoms show larger gaps between brain age estimates and chronological age, making them a bellwether for what prolonged traumatic stress can do to the nervous system, as highlighted in coverage that notes how Researchers from the Icahn School of Medicine at Mount Sinai framed their findings.
I see this group as especially important because their experiences are well documented, from the intensity of their exposure at Ground Zero to the evolution of their health over time. That level of detail allows scientists to tease apart how much of the brain aging signal is driven by PTSD itself versus other factors like respiratory illness, cardiovascular disease, or sleep apnea. The emerging consensus is that while those comorbidities matter, PTSD remains a central driver of the accelerated aging pattern, which strengthens the case for targeted mental health interventions as part of long-term monitoring for trauma-exposed individuals.
From lab findings to clinical red flags
All of this research raises a practical question that I grapple with: how should clinicians and patients use the idea of accelerated brain aging in everyday care? One implication is that PTSD should be treated as a systemic condition that affects the brain and body, not just a cluster of psychological symptoms. When findings suggest that PTSD may contribute to neurobiological aging and increase vulnerability to age-related cognitive disorders, it becomes reasonable to argue for earlier cognitive screening, more aggressive management of cardiovascular risk, and closer attention to sleep and metabolic health in trauma survivors, as underscored by reports that PTSD Linked to Accelerated Brain Aging.
At the same time, I think it is important not to turn “older-looking brain” into a deterministic label that leaves people feeling doomed. Brain age is a probabilistic marker, not a sentence, and there is evidence from other domains that lifestyle changes, effective therapy, and medical treatment can slow or even partially reverse biological aging markers. For PTSD, that means trauma-focused psychotherapy, medications when appropriate, and interventions that reduce oxidative stress and improve sleep could all, in theory, help nudge the brain back toward a healthier trajectory, even if they cannot erase the past.
What I watch for next in PTSD and aging research
Looking ahead, I am watching for studies that connect the dots between MRI-based brain age, epigenetic clocks, and real-world outcomes like daily functioning and quality of life. It is one thing to show that a scan or a blood test looks older than expected, and another to demonstrate that specific treatments can slow that clock and reduce dementia risk. The field is already moving in that direction by combining imaging, molecular markers, and longitudinal follow up in cohorts with PTSD, building on the foundation laid by work that has treated PTSD as a driver of accelerated aging at multiple levels of analysis.
I am also paying attention to how these insights might reshape public health responses to mass trauma events, from terrorist attacks to natural disasters. If we accept that PTSD can speed up brain aging, then early intervention after such events is not just about easing immediate distress, it is about protecting long term neurological health. That perspective reframes investments in mental health services, screening programs, and long term monitoring as strategies to preserve cognitive reserve and delay the onset of age-related disease in populations that have already paid a heavy price.
More from MorningOverview