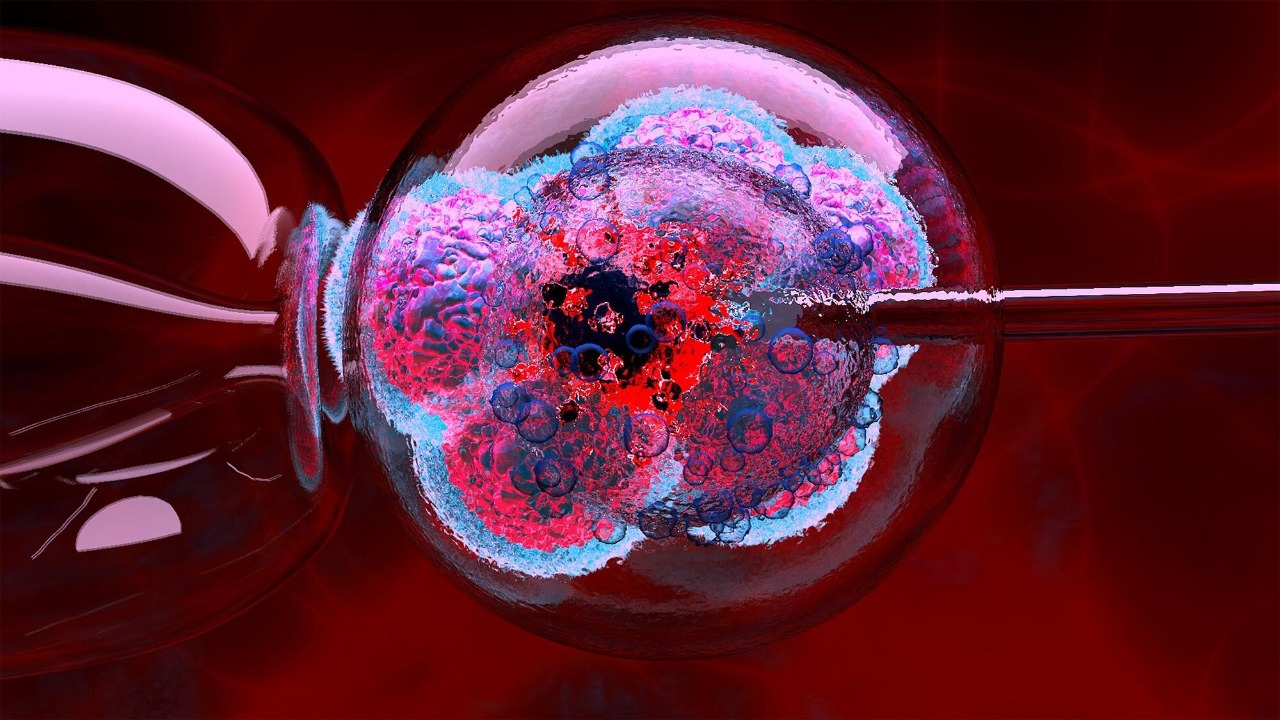
Scientists are edging closer to something that once sounded like science fiction: tools that might refresh aging human eggs and stretch the years in which pregnancy is biologically possible. Instead of only working around the biological clock with egg freezing or IVF, researchers are starting to probe whether the clock itself can be slowed, or even partially rewound, inside the ovary.
That prospect raises profound medical, social, and ethical questions, from how long fertility should be extended to who will benefit first. I want to unpack what this new “egg rejuvenation” work actually involves, how it fits into a broader wave of fertility innovation, and why experts insist that hope needs to be tempered with hard data and careful oversight.
The experimental tool that makes eggs “age” on demand
The most eye catching development is a lab system that lets researchers induce aging like changes in eggs and then test ways to reverse them. Instead of waiting years to see how real oocytes deteriorate, scientists created an experimental setup that triggers the same kinds of molecular and structural problems that accumulate as women get older, including damage to the machinery that keeps chromosomes aligned and separated correctly. By forcing these defects to appear on cue, they can watch in real time how egg quality collapses and which interventions might restore it, a strategy described in detail in work on how Could aging eggs be pushed back toward a more youthful state.
Once those aging like defects are in place, the same platform lets teams test compounds or genetic tweaks that might stabilize the egg’s chromosome separating machinery or repair other age related damage. Early experiments suggest that some interventions can partially restore the structure and function of these artificially aged oocytes, hinting that at least in a dish, aspects of egg aging are not completely irreversible. Follow up reporting on how Scientists ‘rejuvenate’ the aged eggs stresses that these findings are still confined to the lab, but they open a path to targeted drugs or biologics that could one day be tested in people.
Why egg quality collapses long before menopause
To understand why this tool matters, it helps to remember how unforgiving ovarian biology is. Women are born with a finite pool of oocytes, and both the number and quality of those eggs decline steadily over time, which is why fertility drops sharply in the late thirties even though periods may continue for a decade or more. Researchers studying ovarian aging have shown that the structures that keep chromosomes paired and separated cleanly become fragile with age, increasing the risk of errors that can lead to miscarriage or conditions such as trisomies, and the new model system is designed precisely to mimic those failures so they can be dissected and potentially corrected.
That focus on quality, not just quantity, is driving a wave of work on pharmacologic ways to slow ovarian aging. One line of research centers on rapamycin, a drug better known from transplant medicine, which has been proposed as a way to preserve the ovarian reserve and extend the functional lifespan of the ovary by as much as 5 years. Reporting on how a Common drug could extend women’s fertility describes this as an attempt to keep eggs healthier for longer, not to create new ones from scratch.
Rapamycin and the Vibrant trial: slowing the clock from inside
While the egg aging tool operates in a dish, rapamycin is already being tested in living women. The Validating Benefits of Rapamycin for Reproductive Aging Treatment, known by the acronym Vibrant, is designed to see whether carefully dosed rapamycin can preserve ovarian function, reduce the risk of age related diseases, and potentially lengthen the fertile window. The study is not about making someone fertile forever, but about nudging the curve so that the steep drop in egg quality and hormone production happens later, which could translate into a few extra years of reliable ovulation and better IVF outcomes for those who need help conceiving.
Researchers behind The Validating Benefits of Rapamycin for Reproductive Aging Treatment are also watching for broader health effects, since the same pathways that govern ovarian aging intersect with systems involved in metabolism and immune function. Early commentary around Vibrant frames rapamycin as a possible way to align reproductive health with overall longevity, but it also flags the need to balance benefits against side effects that have been documented in other uses of the drug. Coverage of the Vibrant study notes that the trial is explicitly designed to track both fertility measures and the risk of age-related diseases, underscoring how intertwined reproductive aging is with the rest of the body.
From egg freezing to future rejuvenation: how the toolbox is expanding
For now, the main way to hedge against egg aging is to bank younger eggs. Over the past decade, egg freezing has shifted from a niche procedure to a mainstream option for professionals who want to delay parenthood, and experts expect that trend to continue as awareness grows and costs slowly come down. Earlier forecasts about the future fertility treatments landscape predicted that more women will freeze their eggs and that clinics will refine protocols to make stimulation cycles gentler and more efficient.
Even as egg freezing becomes more common, researchers are building tools that could eventually make it one option among many rather than the only serious hedge against the biological clock. A striking example is a new cellular “map” of the ovary that charts how different cell types interact across the lifespan, work that could guide strategies to restore hormone production or even reimplant preserved tissue. The team behind this New ovarian atlas argues that understanding the ovary at single cell resolution is a prerequisite for any serious attempt to extend fertility or safely rejuvenate eggs in situ.
AI, IVF, and the push to make every egg count
While scientists probe how to slow or reverse egg aging, clinics are using artificial intelligence to squeeze more insight out of every IVF cycle. One new system, described as This AI, analyzes embryo images and patient data to predict which transfers are most likely to succeed, with developers claiming it can forecast IVF success 90% of the time. The same platform is paired with a financial model in which patients do not pay if they do not have a child, a sign of how confident its creators are in the 90% prediction rate.
These tools sit on top of a procedure that is already central to modern fertility care. Basics of IVF and Assisted Reproductive Technologies explain how In Vitro Fertilization involves stimulating the ovaries, retrieving eggs, fertilizing them in the lab, and then transferring embryos back into the uterus, a process that has helped millions of families. Clinics now frame IVF as part of a broader ecosystem of technologies that address infertility and reproductive aging, and some highlight how In Vitro Fertilization is being refined alongside genetic testing, time lapse imaging, and AI driven decision support to improve outcomes for older patients whose eggs are already under strain.
The modern dilemma: timing, egg freezing, and the global egg squeeze
Even as the science races ahead, the social pressures that drive demand for these technologies are intensifying. The modern dilemma: Timing vs. Biological clock captures how One of the biggest challenges for today’s generation is reconciling career, housing, and partnership timelines with the steep decline in natural fertility that begins in the early thirties. Clinics that specialize in egg freezing now market the procedure as a way to extend fertility beyond the traditional timeframe, positioning it as a form of reproductive insurance for those who are not ready to start families but do not want to lose their chance.
At the same time, there is a growing recognition that eggs themselves are becoming a scarce resource in some contexts. A preprint titled Navigating the global egg shortage describes how demand for donor eggs, IVF cycles, and fertility preservation is outpacing supply in several regions, creating ethical and logistical challenges for clinics and patients. The authors of Navigating the global egg shortage stress that their analysis should not be used to guide clinical practice, but their framing underscores why technologies that preserve or rejuvenate a woman’s own eggs are so attractive: they could reduce reliance on donor gametes and ease pressure on an already stretched system.
Stem cells, AI, and micro-robots: rebuilding the ovary from the ground up
Beyond drugs and lab models, some teams are trying to rebuild or augment the ovary itself. One ambitious program, described under the banner Developing AI, uses Precision Targeting and Single cell transcriptomics to design stem cell based therapies that could repair or replace aging ovarian tissue. The researchers emphasize that they must overcome immune rejection, ensure safety, and prove long term efficacy before such treatments can move beyond preclinical studies, but they argue that AI guided analysis of ovarian cells can pinpoint exactly which populations need to be restored to maintain hormone production and egg quality, a vision laid out in work on Developing AI driven stem cell therapies.
Stem cell approaches are already being explored in reproductive medicine more broadly, with experimental protocols that aim to regenerate damaged ovaries, uterine lining, or even sperm producing tissue. Overviews of the Treatment of female infertility with stem cells describe this as a promising direction, but they also stress that these methods have yet been widely used clinically and remain confined to research settings or tightly controlled pilot programs. As one summary of Stem cell use in infertility notes, the gap between proof of concept and routine care is still wide, particularly when germ cells and future offspring are involved.
Inside the lab: from testicular cell media to micro/nanorobotics
Some of the most intriguing work is happening far upstream of any clinical application. One study looked at how human testicular cell conditioned media might influence the in vitro development of follicles from cryopreserved human ovarian cortical pieces, essentially asking whether factors secreted by male reproductive cells could coax frozen ovarian tissue to grow healthier follicles in the lab. The authors are explicit that None of these have yet reached clinical trials and that many ethical aspects have yet to be addressed, but they suggest that In the future, such approaches might be part of a toolkit to restore fertility in oncological patients whose ovaries were damaged by treatment, a possibility outlined in their clinical trial oriented discussion.
On a very different front, engineers are experimenting with micro and nanorobotics to assist with fertilization itself. Tiny magnetic or chemically driven devices can be guided to transport sperm, manipulate eggs, or deliver drugs directly to reproductive tissues, potentially making IVF less invasive or more efficient. A review of Micro/Nanorobotics in In Vitro Fertilization argues that In the future, ongoing investigations and advancements in this domain hold the potential to significantly transform reproductive medicine by attempting to surmount obstacles related to fertility, including poor sperm motility or difficult egg retrievals. The authors of In the review caution that these devices are still experimental, but they see them as part of a broader shift toward precision micromanipulation of gametes and embryos.
What clinics are doing now, and why “rejuvenation” is still experimental
Against this backdrop of futuristic research, day to day fertility care remains grounded in more familiar tools. Many clinics emphasize that Ongoing Research Research plays a vital role in advancing fertility treatments, but their current offerings still revolve around IVF, intrauterine insemination, hormone therapies, and egg or embryo freezing. One center describes how its team actively participates in clinical trials and evaluates new technologies and medications that can enhance patient outcomes, but it also makes clear that only interventions with solid safety and efficacy data are incorporated into routine care, a stance reflected in its Ongoing Research Research policy.
Academic reviews back up that caution. A comprehensive assessment of emerging strategies to mitigate reproductive aging concludes that there are currently no practical therapies that have been shown to extend human reproductive lifespan in a reliable, clinically validated way. The authors write in their Conclusions that There is growing momentum behind efforts to reverse reproductive aging, but they stress that most interventions are still in preclinical stages or early phase trials and that long term safety data are lacking. Their analysis, summarized in Conclusions, serves as a reminder that “egg rejuvenation” remains a research goal, not a service anyone can book at a clinic.
Ethics, expectations, and the road from lab bench to bedside
When it comes to ovarian insufficiency and related conditions, even the most cutting edge interventions are still labeled experimental. A detailed review of Novel Approaches in Addressing Ovarian Insufficiency in 2019 notes that this very fact renders all of them to be at an experimental stage rather than of established clinical routine, and that improving live birth rates should be the main objective in published literature. That sober framing is important, because it pushes back against hype and reminds both clinicians and patients that promising lab results do not automatically translate into safe, effective treatments, especially when germline cells and future children are involved, a point underscored in the Novel Approaches review.
Some of the most headline grabbing ideas, such as transferring components from younger, healthier eggs into the eggs of older women, illustrate both the promise and the peril of this field. Reports on infertility research note that Some studies are exploring whether transferring components from younger, healthier eggs into the eggs of older women could improve egg quality, but they also emphasize that such techniques are not yet available as standard treatment options. The summary on Some infertility treatment trials highlights that regulators and ethicists will need to weigh potential benefits against concerns about genetic identity, intergenerational effects, and equitable access long before egg rejuvenation moves from the lab bench to the bedside.
More from MorningOverview