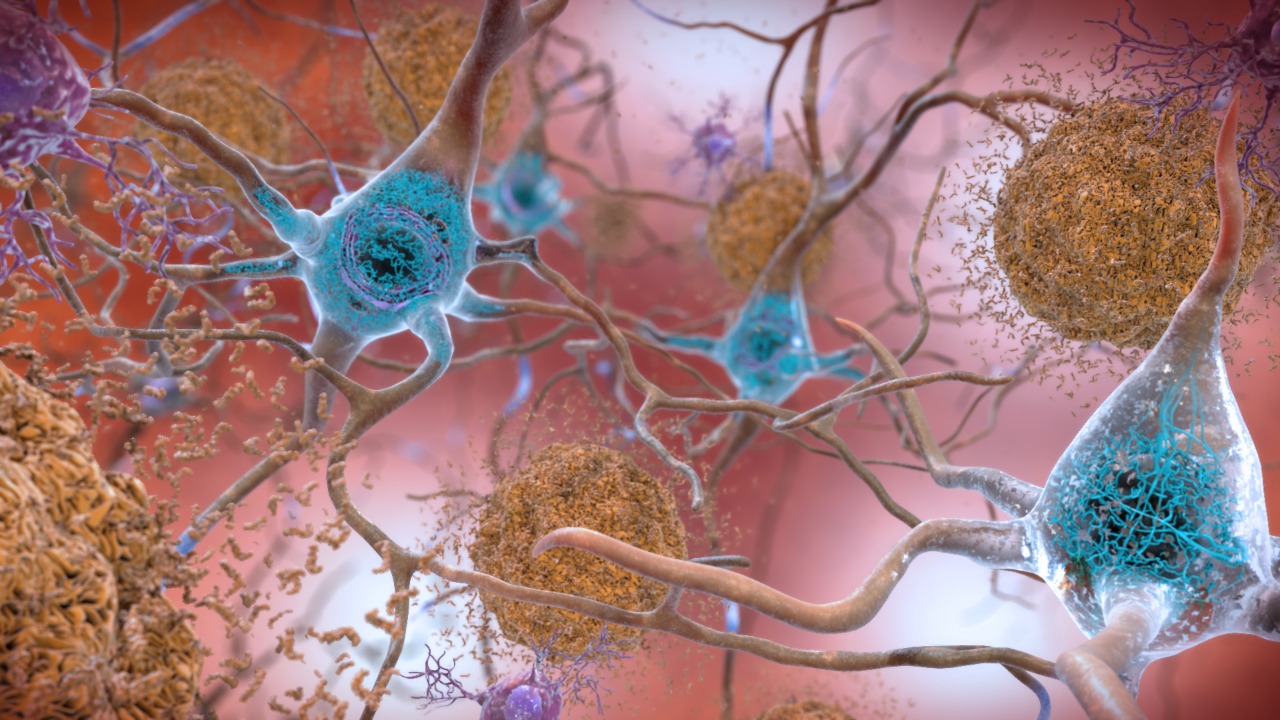
A recent study published in October 2025 has unveiled a surprising potential for amyloid-beta, a protein typically associated with Alzheimer’s disease, to combat cancer. Researchers at the University of California, San Diego, discovered that amyloid-beta aggregates can significantly reduce tumor cell proliferation, achieving a 70% reduction in cancer cell growth in preclinical models. This finding suggests a dual role for amyloid-beta, bridging treatments for neurodegenerative diseases and oncology [source].
The Role of Amyloid-Beta in Alzheimer’s and Cancer
Amyloid-beta plaques are a hallmark of Alzheimer’s disease, accumulating in the brain and leading to neuronal toxicity and cognitive decline. Interestingly, these same plaques exhibit inhibitory effects on cancer pathways, such as apoptosis resistance in tumor cells. The study from the University of California, San Diego, highlights how amyloid-beta’s aggregation state influences its toxicity in neurons while offering potential as a targeted agent against aggressive cancers like glioblastoma. Lab experiments have shown that amyloid-beta selectively binds to cancer cell membranes, disrupting their growth [source].
Despite its promising anti-cancer properties, harnessing amyloid-beta for therapeutic use presents challenges. Delivery methods must be refined to avoid off-target brain damage, a significant concern given the protein’s role in Alzheimer’s pathology. Early data from mouse models suggest that controlled dosing can minimize Alzheimer’s-like symptoms, indicating a potential pathway for safe application in cancer treatment [source].
Insights from Neuronal Metabolism in Alzheimer’s Research
Disruptions in glycogen metabolism within neurons have been identified as a key factor in Alzheimer’s progression. A study from July 2025 found that impaired energy production exacerbates protein misfolding, a critical vulnerability in the disease’s development. This insight opens avenues for metabolic therapies targeting glycogen pathways, which could also benefit cancer treatment by reducing systemic inflammation and supporting anti-tumor mechanisms [source].
Preclinical evidence suggests that enhancing glycogen synthase activity in brain cells can delay amyloid-beta buildup, providing a foundation for dual-purpose drugs. These drugs could potentially sensitize cancer cells to protein-based therapies, offering a novel approach to treating both Alzheimer’s and cancer. By restoring neuronal energy balance, these therapies may indirectly support anti-cancer strategies, highlighting the interconnectedness of metabolic and protein-targeting interventions [source].
Emerging Therapeutic Approaches from Alzheimer’s Breakthroughs

Research into lithium as a potential Alzheimer’s treatment at Harvard Medical School has shown promise. Low-dose lithium has been found to modulate tau protein phosphorylation, slowing disease advancement without significant side effects in early trials. This neuroprotective effect could extend to cancer treatment by stabilizing amyloid-beta levels, preventing excessive toxicity while promoting its use in oncology to inhibit metastasis in breast cancer cell lines [source].
The integration of lithium into broader Alzheimer’s strategies is supported by global collaborations accelerating clinical trials for metabolic and protein-targeting interventions. A report from the World Economic Forum in June 2025 highlights these efforts, emphasizing the potential for lithium to play a role in both neurodegenerative and cancer therapies. This collaborative approach underscores the importance of cross-disciplinary research in developing effective treatments [source].
Protective Proteins and Preventive Strategies
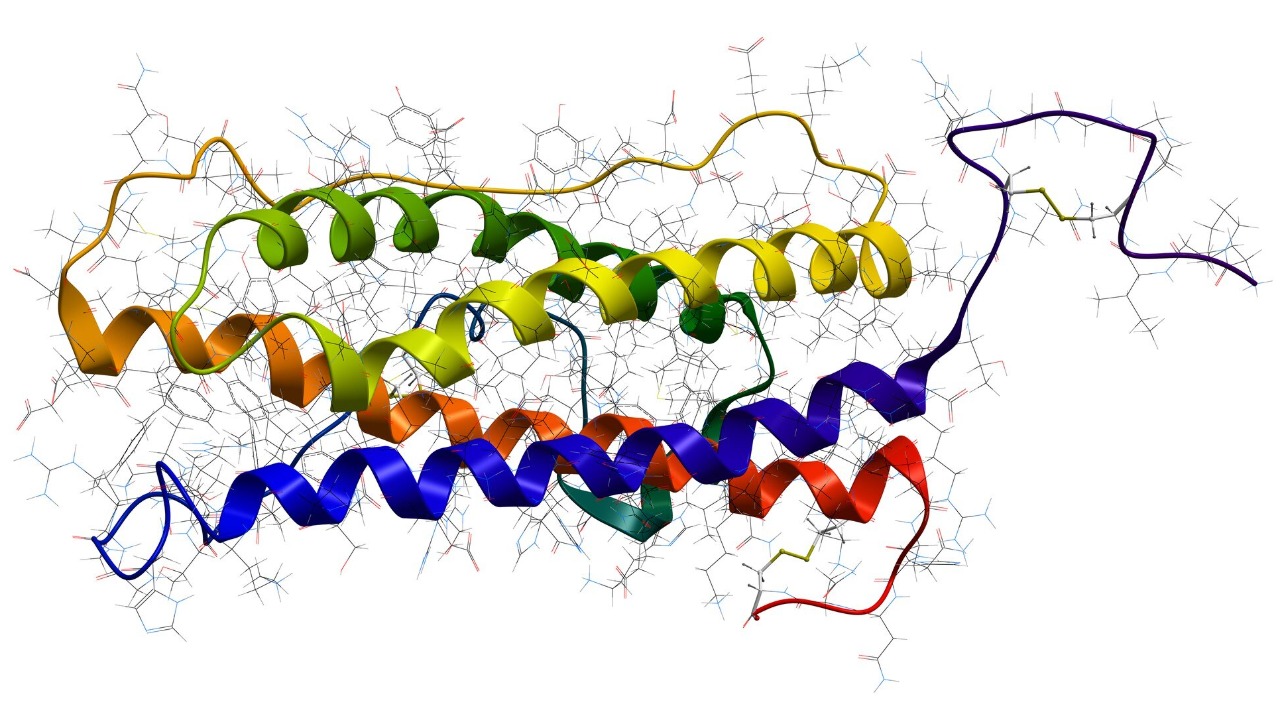
The midkine protein has emerged as a significant player in preventing Alzheimer’s disease, as identified by St. Jude Children’s Research Hospital. Midkine reduces amyloid-beta-induced inflammation in the brain, lowering Alzheimer’s risk in animal models by 50%. This protein’s anti-inflammatory properties could also enhance amyloid-beta’s tumor-suppressing actions, particularly in pediatric cancers like neuroblastoma [source].
Ongoing research at St. Jude focuses on midkine gene therapy to bolster preventive effects against Alzheimer’s. This approach has implications for combination treatments that balance neuroprotection and anti-cancer efficacy. By mitigating toxicity and enhancing tumor suppression, midkine could play a crucial role in developing comprehensive treatment strategies for both Alzheimer’s and cancer [source].