Researchers at the University of Pennsylvania’s Perelman School of Medicine have made a groundbreaking discovery that could revolutionize chronic pain treatment. They have identified specific neurons in the brainstem’s rostral ventromedial medulla (RVM) that act as a potential “off switch” for chronic pain signals. By activating these “off” cells, pain in mouse models was suppressed without affecting normal sensation or causing side effects. This study, led by Jina Wang and published in Science Advances, demonstrates that optogenetically stimulating these neurons reduces pain behaviors in mice with chronic inflammatory pain. This breakthrough offers hope for the estimated 50 million Americans suffering from chronic pain, providing a targeted approach that avoids the addiction risks associated with opioids (Penn Today, SciTechDaily, BBC).
The Brain’s Pain Regulation Mechanism
The rostral ventromedial medulla (RVM) in the brainstem plays a crucial role in pain regulation, containing two main types of neurons: “on” cells that amplify pain signals and “off” cells that inhibit them. The study revealed that “off” cells specifically target pain pathways to provide descending inhibition, offering a new perspective on managing chronic pain (Penn Today). In chronic pain conditions, an imbalance occurs, favoring “on” cells. However, activating “off” cells restores suppression, as demonstrated through single-cell RNA sequencing that identified molecular markers for these neuron populations (SciTechDaily).
Normal pain serves a protective role, alerting us to potential harm, but chronic pain persists due to altered brainstem activity. The research emphasizes that activating “off” cells does not impair acute pain responses needed for injury avoidance. This distinction is crucial, as it suggests that the therapeutic activation of “off” cells could alleviate chronic pain without compromising the body’s natural defense mechanisms (Popular Mechanics).
Experimental Methods and Results
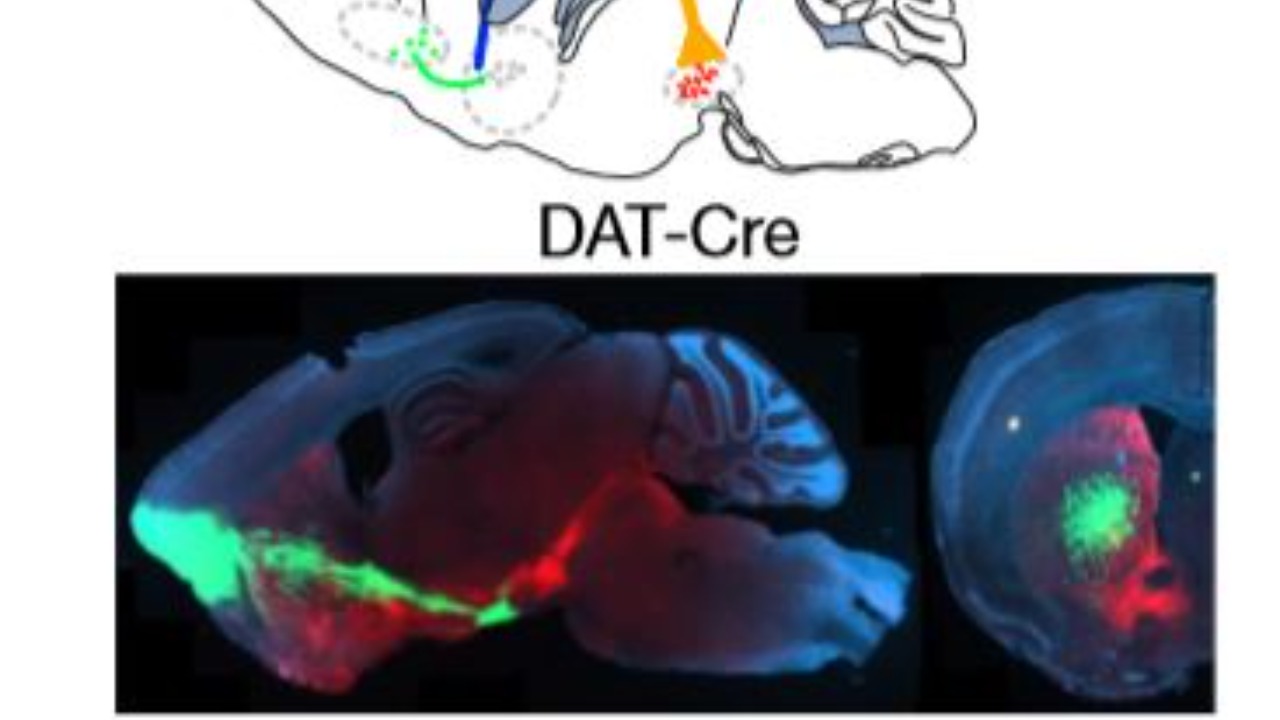
The research team employed optogenetics to precisely activate RVM “off” cells in mice with induced chronic inflammatory pain via hind paw injection. This method resulted in a significant reduction in pain hypersensitivity, as measured by mechanical and thermal tests. The findings underscore the potential of targeting specific neurons to modulate pain without broader neurological impacts (Penn Today).
Through single-cell transcriptomics on over 10,000 RVM neurons, researchers classified “off” cells by their expression of genes like Pdyn and Sst, confirming their role in pain modulation. This approach not only highlights the specificity of “off” cells in pain regulation but also opens avenues for developing targeted therapies that minimize side effects (SciTechDaily).
In behavioral assays, mice with activated “off” cells showed normalized withdrawal thresholds comparable to healthy controls, demonstrating a reversal of allodynia and hyperalgesia specific to chronic states. This result is promising for future applications in human pain management, suggesting that similar interventions could alleviate chronic pain symptoms effectively (BBC).
Potential Therapeutic Applications

Targeting RVM “off” cells could pave the way for developing non-opioid drugs or neuromodulation devices that selectively enhance pain suppression. This approach addresses the limitations of current treatments, which often lead to tolerance or addiction. The discovery of these neurons’ molecular signatures could accelerate the development of safer analgesics, offering a more precise method of pain management (Popular Mechanics).
The research also opens avenues for gene therapy or deep brain stimulation focused on brainstem neurons. Such interventions could benefit conditions like neuropathic pain from diabetes or injury, which affect approximately 20 million U.S. adults. Gregory Scherrer, a leading figure in the study, noted, “This is a major step toward precision pain medicine,” emphasizing the potential impact of these findings on future treatment strategies (SciTechDaily).
Limitations and Next Steps
While the findings are promising, translating “off” cell activation to humans requires further validation. Brainstem targeting poses surgical risks, and individual variability in pain pathways could affect treatment efficacy. These challenges highlight the need for careful consideration in developing human applications (BBC).
The study did not address all chronic pain types, such as fibromyalgia, and future research must explore the long-term effects of repeated neuron stimulation to ensure no compensatory mechanisms emerge. Addressing these gaps is essential for creating comprehensive treatment options that cater to diverse chronic pain conditions (Popular Mechanics).
Ongoing work at the University of Pennsylvania aims to screen compounds that mimic “off” cell activity, with clinical trials potentially feasible within 5–10 years if preclinical safety is confirmed. This timeline reflects the cautious optimism surrounding this research, as scientists continue to explore the full potential of these findings in clinical settings (Penn Today).