Researchers at an undisclosed institution have achieved a groundbreaking development in the field of nanotechnology by creating the world’s first fully functional mirror-image nanopore using D-amino acids. This innovation, reported in October 2025, mirrors natural protein structures but with reversed chirality, allowing it to evade biological degradation. The breakthrough promises to revolutionize biomedical applications by enabling more stable sensors and drug delivery systems resistant to enzymatic breakdown. This technology specifically paves the way for advanced cancer treatments through targeted therapies that could bypass immune responses and improve efficacy (Phys.org).
The Science Behind Mirror-Image Nanopores
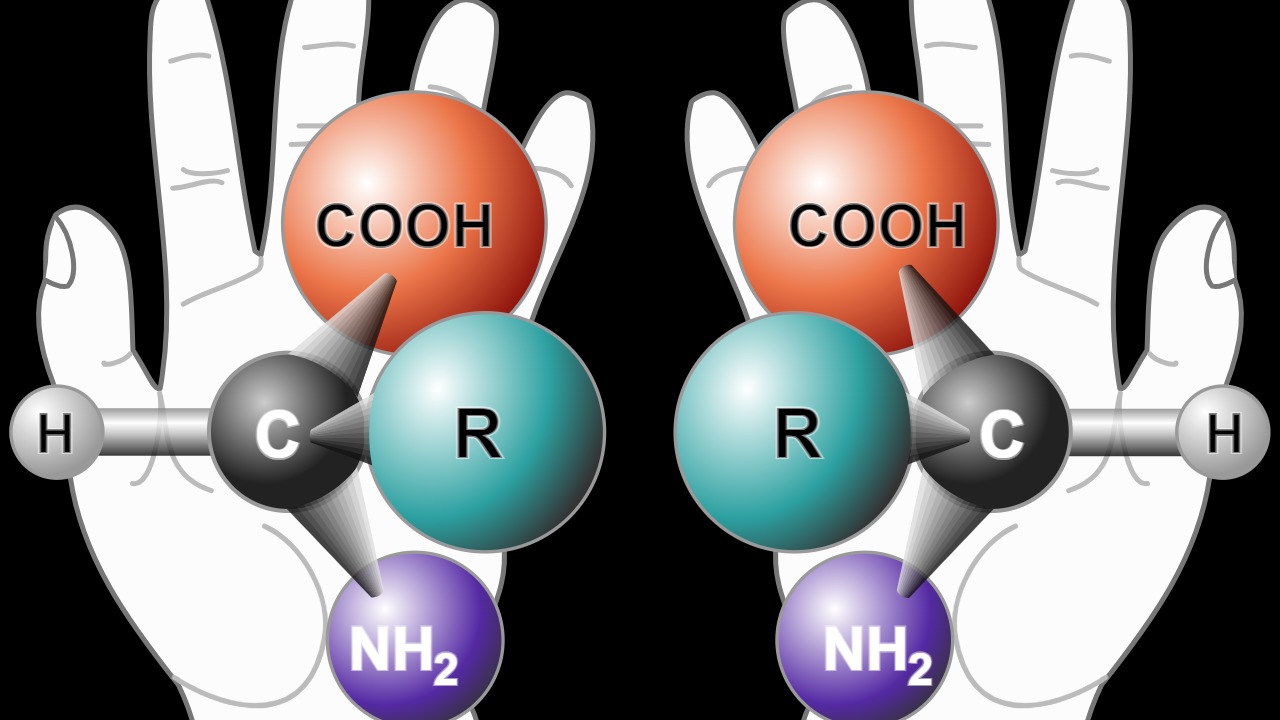
Chirality is a fundamental concept in chemistry, particularly in the structure of proteins. Natural proteins are typically composed of L-amino acids, which form left-handed structures. In contrast, D-amino acids create right-handed mirror images that are resistant to protease enzymes in the body. This resistance is crucial because it allows the mirror-image nanopores to maintain their structure and function in biological environments without being degraded by the body’s natural processes (SSBCrack News).
The fabrication process of these nanopores involves sophisticated synthetic biology techniques. Researchers assemble the D-amino acid-based structures to function identically to their natural counterparts but with enhanced stability. This process ensures that the nanopores can perform their intended functions over extended periods without losing integrity, a significant advancement over traditional methods (Phys.org).
Experimental validation has shown that these nanopores can selectively transport ions and molecules, effectively mimicking biological channels. This capability is crucial for applications in biosensing and drug delivery, where precise control over molecular transport is essential. The nanopores’ ability to maintain functionality without degradation over time highlights their potential for long-term biomedical applications (SSBCrack News).
Advantages Over Traditional Nanopores
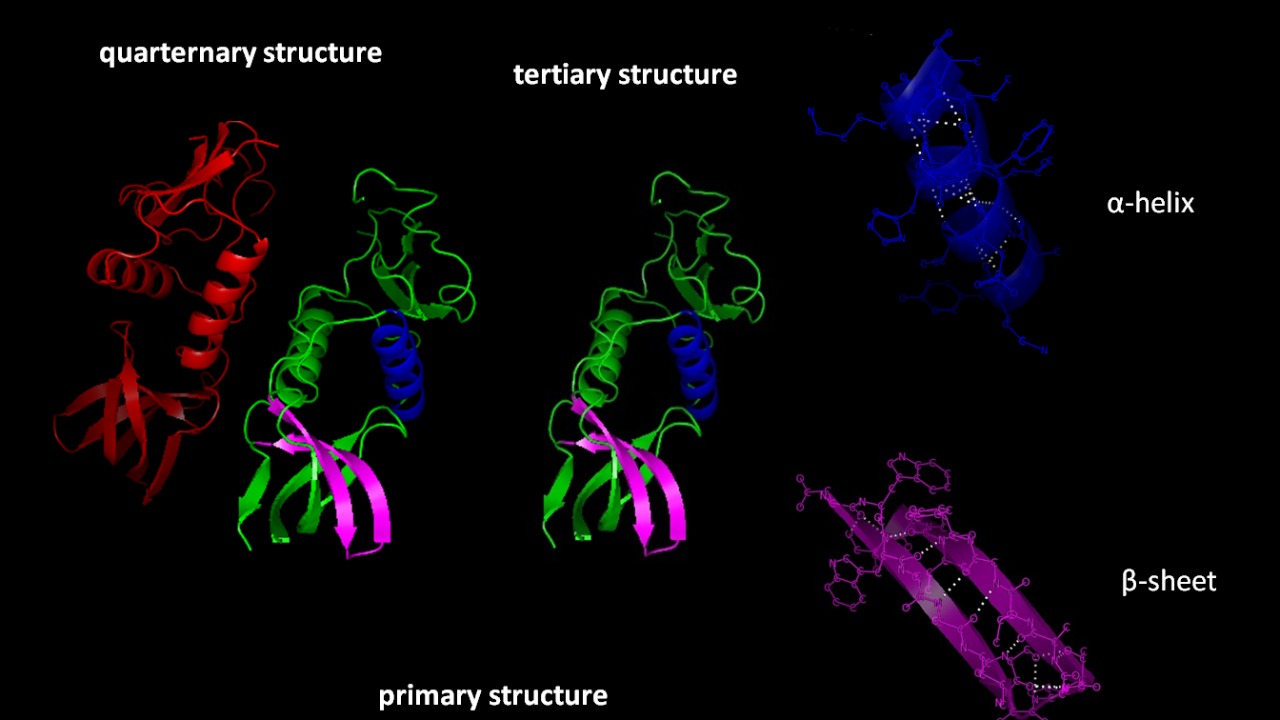
One of the primary advantages of mirror-image nanopores is their ability to avoid recognition and breakdown by natural enzymes. This characteristic significantly extends their lifespan in physiological environments compared to nanopores made from L-amino acids. The increased durability of these structures makes them ideal for applications that require prolonged stability, such as in vivo sensors and drug delivery systems (Phys.org).
Moreover, the reversed chirality of these nanopores enhances their biocompatibility by reducing unwanted immune interactions. This property is particularly beneficial for long-term implants, as it minimizes the risk of immune rejection and inflammation. Such improvements in biocompatibility are crucial for the development of medical devices that need to function seamlessly within the human body (SSBCrack News).
Performance metrics for mirror-image nanopores also show significant improvements over traditional versions. They exhibit higher throughput rates and selectivity in detecting specific biomolecules, which is essential for applications in diagnostics and therapeutic monitoring. The ability to operate without interference from cellular machinery further underscores their potential in precise biomedical applications (Phys.org).
Biomedical Applications in Cancer Therapy
The potential applications of mirror-image nanopores in cancer therapy are particularly promising. These nanopores can be used for targeted drug delivery, encapsulating chemotherapy agents and releasing them directly at tumor sites. This targeted approach minimizes side effects by reducing the exposure of healthy tissues to toxic drugs, thereby improving patient outcomes (SSBCrack News).
In addition to drug delivery, mirror-image nanopores have potential diagnostic uses. They can be employed in real-time sensing of cancer biomarkers through their selective ion channels. This capability enables earlier detection of cancer and the development of personalized treatment plans, which are crucial for improving survival rates and treatment efficacy (Phys.org).
Furthermore, these nanopores can be integrated with immunotherapy strategies. Their stability allows for the delivery of mirror-image peptides that enhance T-cell responses against tumors. This integration could lead to more effective immunotherapies, providing a powerful tool in the fight against cancer (SSBCrack News).
Challenges and Future Developments
Despite their potential, there are challenges in scaling up the production of D-amino acid nanopores to clinical volumes. Optimizing synthesis methods to reduce costs is essential for making these technologies accessible for widespread medical use. Addressing these scalability issues is a critical step toward realizing the full potential of mirror-image nanopores in healthcare (Phys.org).
Regulatory hurdles also pose significant challenges. Before these nanopores can be used in human treatments, long-term safety must be demonstrated in animal models. This requirement ensures that the nanopores are safe and effective for human use, a necessary step for gaining biomedical approval (SSBCrack News).
Ongoing research is exploring hybrid nanopores that combine D- and L-amino acids to create structures with tunable properties. These developments could expand the applications of nanopores beyond cancer therapy, potentially impacting a wide range of biomedical fields. As research progresses, the versatility and functionality of these innovative structures are expected to grow, opening new avenues for medical advancements (Phys.org).