Recent analysis of MRI scans from 2,164 cognitively healthy adults aged 55 to 90, drawn from the UK Biobank, has revealed a perplexing paradox in the realm of neuroscience. Men’s brains undergo faster age-related shrinkage, particularly in regions like the hippocampus and amygdala, yet women are nearly twice as likely to develop Alzheimer’s disease after age 65. This disparity has left neuroscientists grappling for explanations and underscores the complex interplay between brain aging and dementia risk.
The Study Behind the Brain Shrinkage Findings
The research, led by Dr. Lisa Silbert from Oregon Health & Science University, examined longitudinal MRI data from the UK Biobank cohort, tracking brain volume changes over an average of 3.2 years in participants without cognitive impairment. The study measured atrophy in specific brain regions, finding that men experienced greater volume loss in the total brain (-0.49% annually), cerebral white matter (-0.78%), and subcortical gray matter (-0.65%), while women’s losses were slower across these areas. source
Interestingly, no significant sex differences were observed in ventricular expansion rates. This suggests that the faster shrinkage in men is not simply due to overall head size variations, adding another layer of complexity to the findings.
Dissecting the Alzheimer’s Prevalence Gap

Epidemiological data indicates that women account for about two-thirds of Alzheimer’s cases in the United States, with a lifetime risk at 1 in 5 for women over 65 compared to 1 in 10 for men. This higher incidence in women persists even after adjusting for longevity, as women outlive men on average but show elevated dementia rates independent of lifespan differences. source
The paradox is particularly evident in the hippocampus, a key Alzheimer’s-affected region, where men show faster shrinkage (-0.72% per year) yet lower disease rates than women. This discrepancy between brain atrophy and Alzheimer’s prevalence continues to puzzle scientists.
Hormonal Influences on Brain Aging
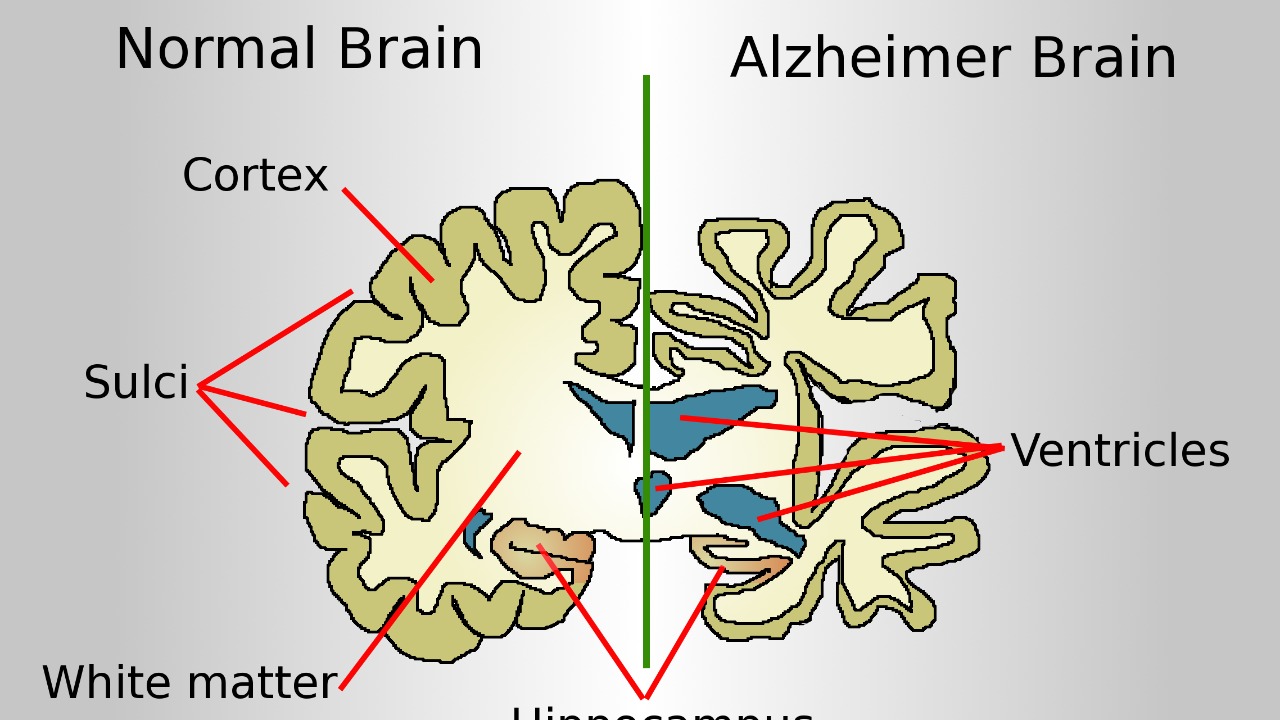
One potential explanation for these findings could lie in hormonal influences. Estrogen’s protective effects in premenopausal women may slow brain atrophy, but post-menopausal declines in estrogen levels could contribute to the heightened Alzheimer’s vulnerability in older women. However, this does not fully account for the shrinkage disparity. source
On the other hand, testosterone in men might accelerate certain atrophy processes, as evidenced by faster losses in male-specific brain regions. Yet, its role in protecting against Alzheimer’s remains unclear, adding another piece to the puzzle.
Lifestyle and Genetic Factors in the Puzzle

Differences in cardiovascular health, with men facing higher midlife risks from factors like smoking and hypertension, may drive faster brain shrinkage. Yet, these do not translate to higher Alzheimer’s rates in men. The APOE4 gene variant, a major Alzheimer’s risk factor, appears to interact differently by sex, conferring greater risk in women, potentially explaining part of the gap despite men’s atrophy advantage. source
Education and cognitive reserve differences, where women often have higher educational attainment in recent cohorts, might buffer against dementia symptoms, but this does not address the underlying atrophy rates. These lifestyle and genetic factors add more variables to the already complex equation of brain aging and Alzheimer’s risk.
Limitations of Current Brain Imaging Research
While the study provides valuable insights, it comes with its limitations. The UK Biobank sample, while large, primarily includes white, middle-aged to older adults from the UK, limiting generalizability to diverse populations or earlier life stages where sex differences might emerge. source
Additionally, short follow-up periods of about 3 years in the study may miss long-term trajectories, and the focus on cognitively healthy individuals excludes insights into how atrophy progresses in those nearing Alzheimer’s onset. These limitations highlight the need for further research in this field.
Future Directions for Unraveling the Paradox

Future research directions could include longitudinal studies incorporating diverse ethnic groups and tracking from midlife. This could clarify if men’s faster shrinkage predicts other neurodegenerative outcomes beyond Alzheimer’s. Integrating genetic, hormonal, and lifestyle data with advanced imaging may help pinpoint why women’s brains resist atrophy better but succumb more to Alzheimer’s pathology. source
Dr. Silbert emphasized the need for sex-specific interventions, noting, “Understanding these differences is crucial for tailoring prevention strategies,” to address the unexplained gap effectively. As we continue to unravel the complexities of brain aging and Alzheimer’s, these findings underscore the importance of considering sex differences in our quest for effective prevention and treatment strategies.