Scientists from the University of East Anglia (UEA) and Oxford Biodynamics have achieved a significant breakthrough by developing the world’s first blood test capable of diagnosing myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) with 96% accuracy. This innovative test analyzes metabolic profiles in blood samples, distinguishing ME/CFS patients from healthy individuals and those with related conditions like long COVID. The development, detailed in a study published in the journal Cell Reports Medicine, promises to transform diagnosis times from years to mere hours, offering hope to the estimated 250,000 people affected by ME/CFS in the UK alone. Interesting Engineering, Technology Networks, and Sky News report on this groundbreaking advancement.
Understanding ME/CFS
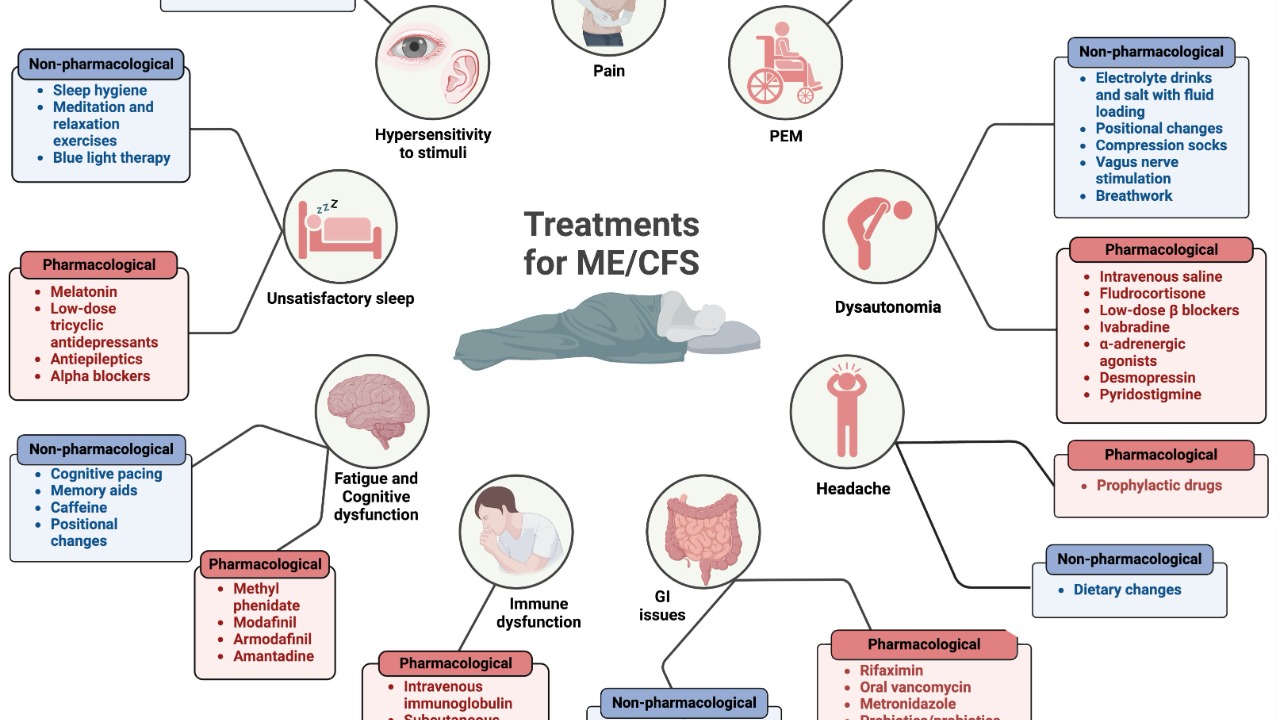
ME/CFS is characterized by a range of debilitating symptoms, including profound fatigue lasting at least six months, post-exertional malaise, unrefreshing sleep, and cognitive impairments. These symptoms have historically made diagnosis challenging, as it relied heavily on clinical criteria without objective biomarkers. This has often led to misdiagnosis or dismissal of the condition as a psychological issue. The lack of a definitive diagnostic test has been a significant barrier, leaving many patients without a clear diagnosis for years LBC.
In the United States alone, ME/CFS affects up to 2.5 million people and remains without a cure. The condition’s impact is profound, often leading to significant lifestyle changes and challenges in daily functioning. The absence of a single definitive test has meant that diagnosis is typically a process of exclusion, ruling out other illnesses, which can delay confirmation for years Yahoo News. This new blood test could significantly alter this landscape by providing a reliable and quick diagnostic tool.
The Science Behind the Blood Test
The newly developed blood test employs nuclear magnetic resonance (NMR) spectroscopy to measure 37 metabolites in blood serum, identifying a distinct biomarker signature for ME/CFS. This methodology allows for a precise distinction between ME/CFS patients and healthy individuals, as well as those with other conditions like multiple sclerosis or depression. In a study involving 154 participants, the test achieved 96% accuracy in distinguishing ME/CFS from healthy controls and 94% from other conditions Technology Networks.
Moreover, the test has demonstrated the ability to differentiate ME/CFS from long COVID, identifying overlapping but distinct metabolic profiles in 20% of long COVID patients who met ME/CFS criteria. This capability is crucial as it highlights the test’s potential to address the diagnostic challenges posed by conditions with similar symptoms LBC. The precision of this test could pave the way for more targeted treatment approaches and better patient outcomes.
Development and Key Contributors
The development of this groundbreaking test was led by Dr. Federica Randi from UEA’s Norwich Medical School, alongside a team at Oxford Biodynamics. The project, initiated in 2019, utilized the EagleBio platform for biomarker discovery. This collaborative effort involved the ME/CFS Research Group at UEA and received funding from the ME Association and Invest in ME Research charity Interesting Engineering.
Dr. Glen Fox, CEO of Oxford Biodynamics, emphasized the test’s potential impact, stating, “This is a game-changer for patients who have struggled for recognition and diagnosis.” His statement underscores the significant step forward this test represents for clinical use and patient care Technology Networks. The collaboration between academic and industry partners highlights the importance of interdisciplinary efforts in advancing medical diagnostics.
Potential Impact on Patients and Research

The introduction of this blood test could dramatically reduce diagnostic delays, enabling faster access to management strategies such as pacing and symptom relief for the 17-24 million people worldwide affected by ME/CFS. This advancement not only promises to improve patient outcomes but also validates ME/CFS as a biological illness rather than a psychosomatic condition, potentially accelerating drug development and therapeutic research Sky News.
Looking ahead, the next steps include plans for larger clinical trials and seeking regulatory approval to make the test available in clinics within the next few years. These efforts will be crucial in ensuring that the test can be widely adopted and integrated into standard diagnostic practices, offering hope and clarity to millions of patients worldwide Interesting Engineering. The potential for this test to transform the landscape of ME/CFS diagnosis and treatment is immense, marking a new era in understanding and managing this complex condition.