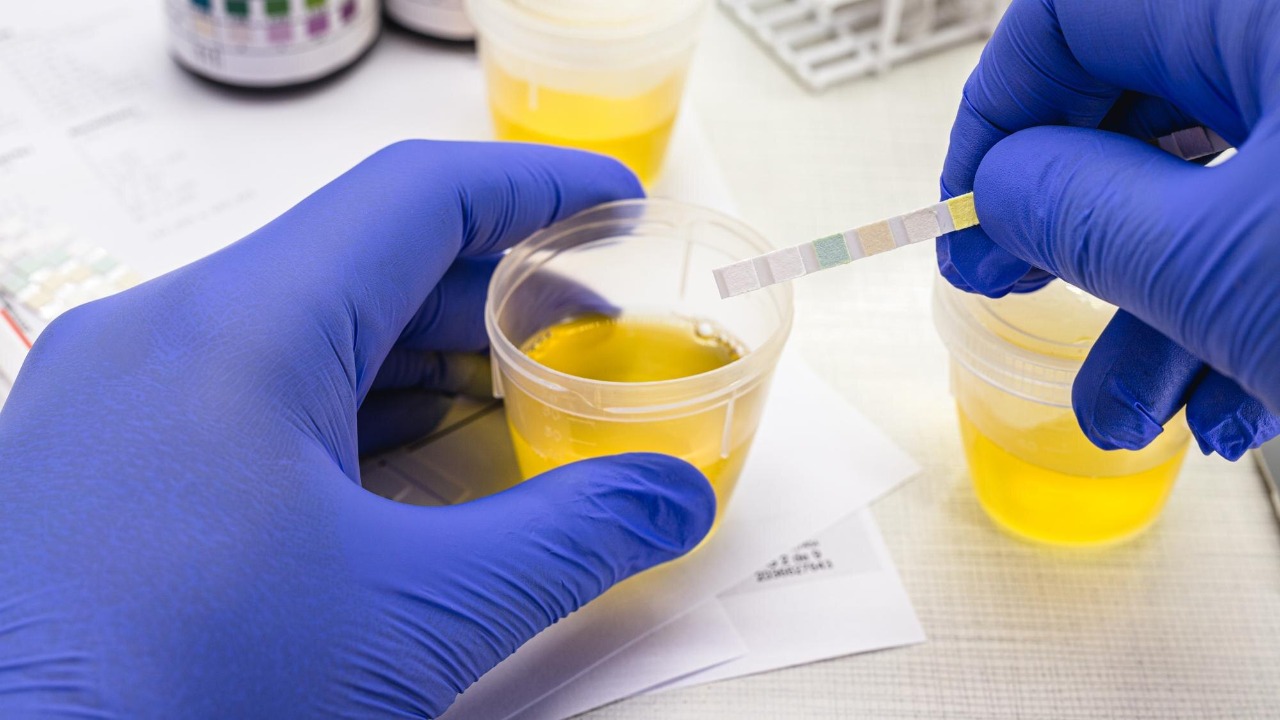
A new generation of aging tests is moving out of specialist labs and into everyday life, promising to tell you how old your body really is, not just how many birthdays you have had. The latest claim comes from a urine-based “aging clock” that reads molecular traces in a simple sample and turns them into a biological age score. Instead of a blood draw or a cheek swab, the pitch is that a quick trip to the bathroom could soon reveal whether your lifestyle is slowing time or quietly speeding it up.
Behind that promise is a convergence of epigenetics, artificial intelligence and decades of work on how aging leaves fingerprints in our cells. I see the urine test as the most accessible expression yet of a broader shift, in which companies and researchers are racing to quantify the pace of aging and sell that number back to us as a new kind of vital sign.
From birthday candles to biological clocks
Chronological age is simple arithmetic, but the body does not age in lockstep with the calendar. Two people who are both 50 can have very different risks of heart disease, dementia or frailty, which is why scientists have spent years searching for biomarkers that capture “physiologic age” instead. One influential line of work has focused on DNA methylation patterns, which change in predictable ways over time and underpin epigenetic clocks such as those used by commercial tests like myDNAge.
These epigenetic tools, built on what is known as the Steve Horvath Epigenetic Aging Clock, use machine learning to map methylation at hundreds of thousands of sites and convert that pattern into an age estimate. The company behind myDNAge describes its assay as a way to “precisely estimate your biological age” from a small amount of blood or urine, positioning it as a step beyond ancestry-style DNA kits that simply read static genetic code. That shift from fixed DNA to dynamic molecular changes is the conceptual bridge that leads directly to the new urine-based aging clocks.
How a urine aging clock actually works
The new urine test is built on the idea that our cells constantly shed molecular debris into bodily fluids, and that those fragments carry information about how fast we are aging. Reporting on the technology describes a model that reads microRNAs, tiny regulatory molecules, that are packaged into protective bubbles called extracellular vesicles and excreted in urine. By training an algorithm on these patterns, researchers have created what they call a urine aging clock that can infer biological age from a single sample, turning the bathroom into a kind of molecular observatory for the body’s internal wear and tear, as described in detail in coverage of how urine can now reveal your biological age.
In practical terms, the test works a bit like a high-tech pregnancy strip, but with a far more complex back end. One account invites readers to “Imagine peeing on a stick” that does not just check for sugar spikes but instead feeds molecular data into an artificial intelligence model that has been trained to distinguish younger from older profiles and to see which lifestyle factors are cranking up the clock. The same reporting notes that gender matters here too, with models adjusted for male and female biology, and that the goal is to flag when cells stop dividing cleanly and start causing chaos, a tipping point that the new urine aging clock is designed to detect.
The Japanese study behind the bold claims
The most detailed description of the science comes from Japanese researchers who built an epigenetic urine aging clock using microRNA signatures. Their model analyzes microRNAs in urinary extracellular vesicles and then uses an algorithm to estimate both biological age and the rate of biological ageing, effectively turning a single sample into a snapshot of how quickly someone is moving along the aging trajectory. According to summaries of the work, the researchers report that their model “outperformed blood-based miRNA and mRNA” approaches, suggesting that urine may actually be a richer or more stable source of aging signals than blood for this specific purpose.
Those performance claims matter because they position the urine test not as a gimmick but as a competitor to more established blood-based clocks. The Japanese team’s findings are framed as evidence that a noninvasive sample can rival or surpass venous draws in predictive power, which is why coverage emphasizes that the model outperformed other microRNA and messenger RNA approaches when tested head to head. The same reporting notes that this urine aging clock could, in principle, be used to estimate how long someone has left to live, a provocative idea that has been highlighted in pieces explaining how a simple urine test could reveal your body’s real age.
What the new test can, and cannot, really tell you
It is tempting to treat a biological age score as a verdict on how long you will live, but the science is more nuanced. The urine aging clock is designed to estimate biological age and the biological ageing rate, not to print an expiration date. Reports that the test could hint at “how long you have left to live” are based on statistical associations between accelerated aging markers and higher risks of age-related disease or earlier mortality, not on a deterministic countdown. In other words, a higher score signals elevated risk, not fate, as explained in coverage of how a new urine test could reveal how long you have left to live.
There are also important caveats about who has been studied so far and how generalizable the results are. A detailed technical summary of the work on a urine-based microRNA clock notes that the Study Design and Population Sampling involved specific cohorts, with age ranges and health profiles that may not capture the full diversity of the general population. The same report explains that by reading aging signals shed into urine, the researchers identified reproducible age-related microRNA shifts, but reproducible in a study population is not the same as universally valid. That is why the authors emphasize their Study Design and Population Sampling as a foundation, not a final word.
Why urine is such a powerful aging mirror
Urine may sound like an unlikely window into aging, but it has long been a workhorse fluid for medical diagnostics. One reason is that it concentrates metabolic byproducts and oxidative damage markers that accumulate as cells burn energy and repair DNA. A comprehensive review of urinary biomarkers of oxidative stress in aging notes that aging is a major risk factor for a range of chronic diseases and that oxidative stress theory links cumulative molecular damage to the development of age-related diseases. The authors report that their findings showed that eight specific urinary biomarkers were associated with age and with the development of age-related diseases, underscoring why urine is such a rich source of aging signals, as detailed in work on urinary biomarkers of oxidative stress.
Earlier research has also singled out particular molecules that rise with age in urine. In one study, scientists found an age-dependent increase in urinary 8-oxo-7,8-dihydroguanosine, often abbreviated 8-oxoGsn, in participants 21 years old and older. The lead author, Jian-Ping Cai, explained that this pattern suggested 8-oxoGsn could serve as a marker of accumulated oxidative damage, and therefore as a potential aging indicator. That work, highlighted in a report that quotes Cai saying “Therefore” to underscore the link between animal and human data, helped establish the conceptual basis for using urine as a noninvasive aging readout, as described in coverage of how a simple urine test could measure how much our body has aged.
From oxidative markers to full-fledged clocks
The leap from a single oxidative stress marker to a full biological clock required more than just better chemistry, it needed better statistics. A detailed research article on urinary 8-oxo-7,8-dihydroguanosine as a potential biomarker of aging starts with a Background section that explicitly calls for a molecular biomarker of physiologic age, as opposed to chronologic age, in clinical medicine. The authors report that in males, the levels of urinary 8-oxoGsn increased with age even after adjusting for age-dependent changes in urinary creatinine, suggesting that the signal was not just a byproduct of kidney function but a genuine aging marker. That work, which framed 8-oxoGsn as a candidate biomarker of aging, laid the groundwork for more complex multi-marker models, as detailed in the urinary 8-oxo-7,8-dihydroguanosine study.
Subsequent work expanded beyond a single molecule to panels of markers and then to microRNAs, which can capture a broader slice of cellular regulation. A separate report on new research shows that a substance indicating oxidative damage increases in urine as people get older and describes how this could be used to estimate biological age and even, in a probabilistic sense, the time frame for our death. That framing, which links oxidative damage to lifespan predictions, foreshadows the current generation of urine clocks that combine dozens or hundreds of signals into a single age estimate, as described in coverage of how new research shows oxidative damage increases with age.
How this fits into the booming biological age industry
The urine aging clock is arriving in a market that is already crowded with biological age products. Epigenetic tests like myDNAge, which is explicitly based on the Steve Horvath Epigenetic Aging Clock, promise to estimate biological age from a small amount of blood or urine and then track how that number changes over time. The company’s own description emphasizes that its test is able to precisely estimate biological age using a small amount of blood or urine, positioning it as a premium option for longevity enthusiasts who want to quantify the impact of diet, exercise or supplements, as outlined in the myDNAge test overview.
More broadly, consumer interest in these tools has been primed by the success of genetic ancestry kits. One analysis notes that you have heard of at-home tests like those from 23andMe and Ancestry, which scan your DNA to provide information about ethnicity and inherited traits, and that a new wave of products is now trying to tell you whether your biological age is above or below average. That shift from static DNA to dynamic aging metrics is framed as a natural next step in the quantified self movement, with companies betting that people will pay to know if their lifestyle is making them biologically younger or older than their peers, as explored in a feature asking how old you really are.
AI, microRNAs and the next diagnostic frontier
What sets the latest urine clock apart is not just the sample type but the computational muscle behind it. Reports on the project explain that scientists built a model that reads microRNAs in urinary extracellular vesicles and then uses artificial intelligence to map those patterns onto age, effectively turning a messy molecular soup into a clean number. One account, headlined “Scientists Just Developed a Urine Test That Reveals Your True Biological Age,” underscores that the key innovation is how the AI comes in to interpret complex data and extract a signal that would be invisible to the naked eye, as described in coverage of how Scientists Just Developed a Urine Test That Reveals Your True Biological Age.
The same reporting notes that the test does more than spit out a single age number, it can also provide clues about your body’s internal state by highlighting which pathways or cell types are contributing most to the accelerated or decelerated aging signal. That kind of granular insight is possible because microRNAs regulate gene expression across many tissues, and their patterns can reflect stress, inflammation or metabolic shifts. By training on large datasets, the AI can learn to associate specific microRNA constellations with particular aging trajectories, turning urine into a surprisingly rich readout of what is happening under the skin, as explained in a follow up that details what the test reveals about your body’s internal state.
Lessons from cancer diagnostics and other urine tests
The idea of using urine microRNAs as diagnostic tools did not emerge in a vacuum. In oncology, researchers have spent years trying to identify bladder cancer markers in urine, exploring DNA mutations, DNA methylation, chromosomal changes and, more recently, microRNAs present in the urine. One technical chapter on bladder cancer markers notes that, Therefore, numerous attempts have been made to find other kinds of markers such as DNA mutations, DNA methylation and more recently microRNAs present in the urine, reflecting a broad shift toward noninvasive molecular diagnostics. That same logic, which treats urine as a convenient liquid biopsy, is now being repurposed for aging clocks, as outlined in work on bladder cancer markers and microRNAs.
These precedents matter because they show that the technical hurdles of isolating and analyzing urinary microRNAs have already been partially solved in other fields. The same extraction methods, sequencing technologies and statistical pipelines that were developed to spot tumors can be adapted to detect aging signatures. In that sense, the urine aging clock is less a radical departure and more an opportunistic spin-off, taking advantage of a diagnostic ecosystem that has already learned how to read complex molecular messages in a humble sample cup.
What this could mean for everyday health decisions
If urine-based aging clocks live up to their promise, they could change how people think about preventive health. Instead of waiting for a cholesterol panel or a blood pressure reading to creep up, someone might track their biological age every few months and adjust their habits in response. The appeal is obvious: a single number that reflects the cumulative impact of sleep, diet, exercise and stress, and that might move in the right direction if you swap late-night emails for an extra hour of rest. That feedback loop is exactly what many longevity clinics and wellness apps are already trying to build around existing epigenetic tests.
At the same time, the psychological and ethical implications are significant. Being told that your biological age is a decade older than your chronological age could be a powerful motivator, but it could also provoke anxiety or fatalism, especially if the score proves stubborn. Regulators and clinicians will need to grapple with how these tests are marketed, how their limitations are communicated and how to integrate them into evidence-based care rather than letting them drift into the realm of biohacking toys. For now, the urine aging clock sits at the frontier of that debate, a vivid example of how quickly the science of aging is moving from the lab bench to the bathroom shelf.
More from MorningOverview