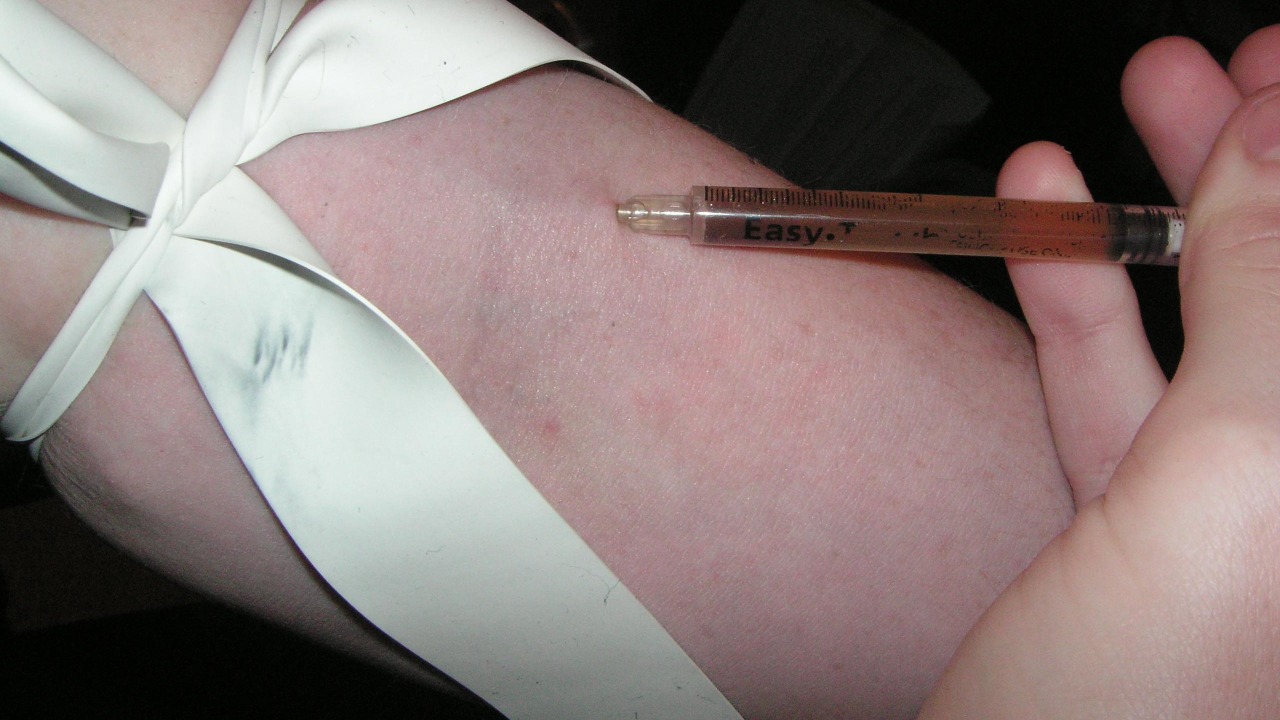
Across social media and encrypted messaging apps, a new frontier of weight loss is unfolding in real time, with people sourcing and injecting an experimental drug that regulators have not yet approved. The rush toward these powerful injections reflects both the desperation created by obesity and the impatience of a culture that has already normalized self-administered shots like semaglutide but is now pushing into far riskier territory.
At the center of this surge is retatrutide, a next‑generation obesity treatment still in clinical trials, which early data suggest can trigger dramatic drops in body weight. Instead of waiting for regulators and doctors, some users are turning to gray and black markets, guided by TikTok influencers and Telegram chats, and treating their own bodies as test sites for a drug that even its developers say is not yet proven safe.
From TikTok hype to Telegram syringes
The new wave of self-injection is not happening in clinics or hospitals, it is unfolding in private kitchens and bedrooms, often after a recommendation from a stranger on TikTok. Influencers showcase shrinking waistlines and before‑and‑after photos, then quietly direct followers to Telegram or WhatsApp groups where unlicensed versions of retatrutide are sold, creating a parallel market that bypasses doctors and pharmacies entirely. In the United Kingdom, head of pharmacy Jason Murphy has warned that these unregulated imports mean people are injecting substances that may not be what the label claims, exposing themselves to potentially serious health risks.
That warning cuts against the frictionless way these drugs are being traded. Buyers wire money through payment apps, receive vials in the mail, then crowdsource dosing advice from other users who have no medical training but speak with the confidence of early adopters. The informality is striking, given that the compound at the center of this frenzy, retatrutide, is still described in official materials as an experimental therapy, not a consumer product, and that even in formal trials participants are closely monitored for side effects that casual users may not recognize until it is too late.
What retatrutide actually is
Retatrutide is not just another version of the now‑familiar GLP‑1 drugs, it is a so‑called triple‑receptor agonist that targets three different hormone pathways involved in appetite and metabolism. In clinical descriptions, The Takeaway is that retatrutide is an experimental drug that has shown promising weight loss results but has not been studied long enough to know that it is safe. That dual reality, powerful effect paired with incomplete safety data, is exactly what makes the current wave of self‑experimentation so fraught.
Unlike semaglutide, which acts on a single GLP receptor, retatrutide is designed to hit GLP‑1, GIP, and glucagon receptors at once, a more aggressive strategy that early studies suggest can drive larger and faster drops in body weight. Yet those same mechanisms can also affect digestion, blood sugar, and cardiovascular function in complex ways that researchers are still mapping. When people inject themselves outside of trials, they are essentially betting that the benefits seen in controlled settings will translate to their own bodies without the safety net of regular lab work, imaging, or physician oversight.
The Ozempic effect and the culture of DIY injections
The willingness of ordinary people to handle syringes and titrate doses at home did not appear overnight, it grew out of the rapid mainstreaming of GLP‑1 drugs like Ozempic and Wegovy. In formal research, Introduction materials on Semaglutide describe it as a GLP receptor agonist peptide, initially approved as a diabetes medicine under the brand Ozempic, that later became a blockbuster weight‑loss treatment. That shift from a specialist diabetes therapy to a household name weight‑loss shot normalized the idea that powerful metabolic drugs could be self‑injected once a week with minimal fuss.
As prescriptions for semaglutide surged, so did off‑label use among communities like online bodybuilders, who traded tips on dosing and stacking it with other compounds to cut fat quickly. Those patterns helped create a cultural template: if a drug can be injected at home and leads to visible changes in body composition, some users will push beyond official guidance and experiment on their own. Retatrutide is now sliding into that template, with people who have already tried GLP‑1 drugs seeing it as the next logical step, even though its safety profile is far less established.
Why retatrutide’s results are so tempting
The appeal of retatrutide is not abstract, it is grounded in numbers that are hard to ignore for anyone who has struggled with obesity. In a phase II trial, the experimental medication appeared to help participants lose nearly one‑third of their body weight, with researchers noting that it seemed more effective than existing GLP drugs because it targets three hormones at once. Reports on this study describe how the experimental medication retatrutide appears to outperform single‑pathway treatments in head‑to‑head comparisons, at least in the short term.
Those headline figures are echoed in company summaries that highlight average weight loss approaching 30 percent of body mass among people who received higher doses. In one overview, Eli Lilly reported that its new drug, retatrutide, resulted in an average weight loss of nearly 30 percent, with some participants achieving over 30 percent weight loss. For someone who has cycled through diets, gym memberships, and older medications with only modest results, those numbers can feel like a once‑in‑a‑lifetime opportunity, which helps explain why some are unwilling to wait for regulators to finish their work.
Inside the lives of early adopters
Behind the statistics are individuals who have turned their bodies into test cases, often with dramatic outcomes. One man, identified only as Craig, took retatrutide through an official Eli Lilly trial and reported losing up to 80 pounds, describing how the drug reshaped his appetite and energy levels. Another account describes how, because of that experience, he became a kind of informal ambassador for the treatment, fielding questions from strangers who had seen his transformation and wanted to know how to get the same injections for themselves.
Not everyone has access to a formal trial, however, which is where the gray market comes in. Some of the people now sourcing retatrutide online say they were inspired by stories like Craig’s and by coverage that notes how users “absolutely swear by it,” even as experts caution that the long‑term safety data are not yet in. One report highlighted how enthusiasts shared their experiences with retatrutide injections while researchers at Indiana University stressed that the drug remains under study. That tension between personal testimony and scientific caution is now playing out in real time across social feeds and group chats.
The safety gap between trials and kitchen tables
In a clinical trial, every dose of retatrutide is tracked, every side effect is logged, and participants can be pulled off the drug if lab results or symptoms raise red flags. Outside that environment, people are injecting compounds of uncertain purity, often at doses borrowed from trial protocols that were never meant to be copied without medical supervision. Pharmacists like Jason Murphy have underscored that unregulated imports can contain incorrect concentrations or contaminants, warning that people are injecting substances that expose them to potentially serious health risks.
Even with pharmaceutical‑grade product, the long‑term effects of triple‑agonist drugs are still being mapped. Official summaries emphasize that retatrutide is experimental and that researchers have not yet studied it sufficiently to know that it is safe, a point spelled out in The Takeaway on what retatrutide is. When people inject themselves at home, they are stepping into that uncertainty without the backup of regular heart scans, liver panels, or gastrointestinal assessments, and without a clear plan for what happens if they develop complications that current science does not yet fully understand.
Other experimental shots waiting in the wings
Retatrutide is not the only next‑generation obesity drug on the horizon, and the broader pipeline helps explain why the current moment feels like a turning point. One emerging contender is Eloralintide, an experimental obesity drug that mimics the hormone amylin to curb appetite. In early reports, Key Takeaways on Eloralintide note that Patient volunteers experienced meaningful weight loss and appetite suppression, suggesting that amylin‑based therapies could soon compete with Wegovy and Zepbound if they clear regulatory hurdles.
Another candidate is a monthly injection developed by California‑based pharma company Amgen, which has completed a phase II trial and is being positioned as a powerful treatment for obesity. Early coverage describes how a new drug from California company Amgen showed promise as a monthly weight‑loss shot, potentially offering a more convenient dosing schedule than weekly injections. As these drugs move through trials, they are likely to generate their own waves of online buzz, and if regulators and clinicians cannot keep pace with public demand, the pattern of self‑experimentation seen with retatrutide may repeat.
How we got here: transformative drugs and rising expectations
The current appetite for experimental injections is rooted in the extraordinary impact of the first generation of GLP‑1 drugs on both health outcomes and public imagination. Clinicians who work with these medications often describe them as transformative, noting that they have given so many people a tool to lose significant weight and improve conditions like type 2 diabetes and sleep apnea. In one widely cited assessment, a researcher explained that More weight loss drugs have been “transformative to say the least,” and that They have given so many people a new sense of control over their health.
That success has raised expectations for what future drugs should deliver. When people see that semaglutide can help them lose 15 percent of their body weight, they start to ask why they should settle for that if a triple‑agonist like retatrutide might deliver nearly 30 percent. The result is a kind of pharmacological arms race, with patients, influencers, and even some clinicians scanning early‑stage trial data for the next big leap. In that environment, the line between cautious optimism and reckless experimentation can blur quickly, especially when social media rewards bold claims and dramatic transformations more than it rewards nuance about side effects or long‑term unknowns.
Beyond fat loss: inflammation, comorbidities, and new frontiers
Part of what makes these drugs so compelling is that they promise more than a smaller number on the scale. Researchers are increasingly interested in how weight‑loss injections might reduce inflammation and improve conditions like fatty liver disease, cardiovascular risk, and sleep apnea. In one recent discussion, a New York physician, Dr. Sue Decotiis, highlighted how Weight loss drugs show promise in reducing inflammation beyond fat burning, while also stressing that experts urge caution because of potential risk according to the researchers.
At the same time, companies are racing to develop oral versions of these therapies that could make them even more accessible. One briefing noted that, Separately, Novo Nordisk is working on an experimental drug called CagriSema, which combines semaglutide with another compound and is being tested as a pill. If such oral treatments reach the market, they could further normalize pharmacologic weight loss and potentially reduce the appeal of risky self‑injection, but they might also expand the pool of people eager to try the most potent new option, whether or not it has full regulatory approval.
The regulatory clock and the impatience gap
For all the excitement around retatrutide, official timelines remain slow by the standards of social media. One clinic‑focused overview notes that Retatrutide is pending FDA approval and may not be available on the market until 2027. For someone watching their friends or favorite influencers post rapid transformations today, that two‑year horizon can feel like an eternity, especially if they are already living with obesity‑related complications.
That impatience gap is where the current wave of self‑injection lives. People are weighing the known risks of staying at a high body weight against the unknown risks of an experimental drug and deciding that the latter feels more tolerable, particularly when framed through glowing testimonials and dramatic photos. As more experimental agents like Eloralintide and the Amgen monthly shot move through trials, regulators and clinicians will have to grapple not only with the science but with the social dynamics that now surround obesity treatment, where a TikTok video can move faster than any formal approval process and where the syringe in someone’s hand may contain a compound that even its creators are still trying to fully understand.
More from MorningOverview