New work in mice is revealing how losing insulation on just a few nerve fibers can throw off the timing of brain signals and unravel complex thought. Instead of needing widespread damage, the research suggests that tiny gaps in this protective coating can be enough to scramble communication between key regions that support memory, attention, and sensory processing.
By tracing how these microscopic breakdowns disrupt the brain’s internal wiring, scientists are starting to connect the dots between subtle cellular changes and the everyday problems people notice first, such as slower thinking, fuzzy recall, or trouble following conversations. I see this shift as crucial, because it reframes cognitive decline not as a vague, global failure but as a precise, fixable breakdown in the brain’s circuitry.
Why brain “insulation” matters more than we thought
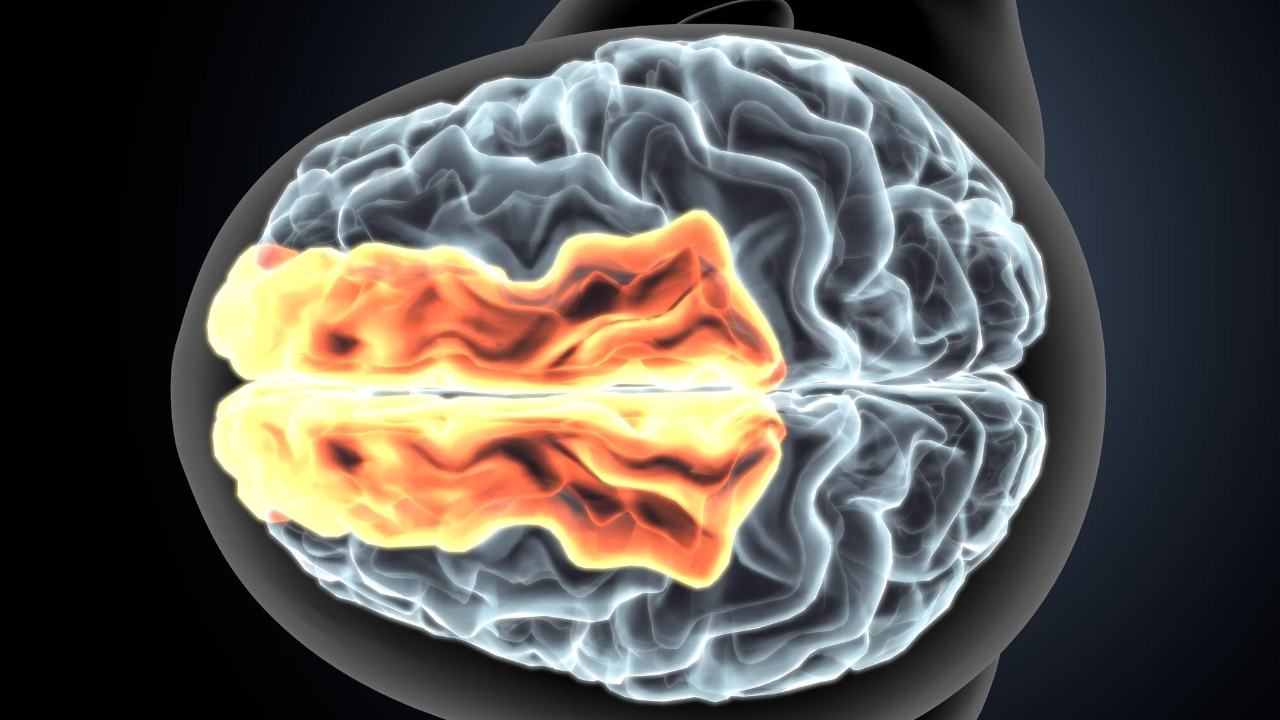
The brain’s wiring depends on myelin, a fatty sheath that wraps around nerve fibers and lets electrical signals travel quickly and in sync. When that insulation is stripped away, even in small patches, the signals that carry thoughts, memories, and sensations can lose their rhythm, arriving too early, too late, or not at all. Researchers have long known that myelin loss is a hallmark of conditions like Multiple Sclerosis, but the new work shows that the scale of damage needed to cause trouble may be far smaller than previously assumed.
Earlier research on ageing brains already pointed to myelin as a central weak point. In one line of work, Scientists reported that the brain’s “wiring insulation” is one of the major factors in age related deterioration, linking the loss of this substance to cognitive decline and diseases such as Multiple Sclerosis. Another study described how a progressive loss of white matter and myelin is a key feature of the ageing brain, and warned that the brain’s ability to replace lost myelin also weakens over time, a point underscored in work on the loss of white matter. Together, these findings set the stage for the latest experiments that zoom in on what happens when only a tiny patch of that insulation disappears.
The mouse experiments that exposed a fragile timing system
To understand how small losses of insulation can have outsized effects, Maarten Kole and colleagues turned to mice and focused on nerve fibers that connect the cortex to deeper brain structures. In their experiments, they selectively disrupted myelin on a limited set of fibers and then watched how the animals’ brain activity and behavior changed. The surprise was not just that the signals slowed, but that the precise timing between different inputs fell apart, undermining the brain’s ability to combine information into a coherent thought.
One report on this work describes how a mouse study revealed that losing a tiny patch of myelin can show up as very human problems, such as trouble remembering familiar names or keeping track of a conversation, even when most of the surrounding tissue looks normal. The researchers found that the affected fibers failed to deliver signals at the exact moment other inputs arrived, which is critical for circuits that depend on coincidence detection. That insight is captured in a detailed account of how Maarten Kole and his group linked tiny structural changes to measurable disruptions in thought-like processes in mice.
Layer 5 myelination and the brain’s coincidence detectors
At the heart of this story is a specific layer of the cortex that sends long range projections to the thalamus, a hub that relays and filters sensory and cognitive signals. The study titled “Layer 5 myelination gates corticothalamic coincidence detection” zeroed in on how myelin along these corticothalamic fibers controls whether the thalamus receives inputs at just the right moment to integrate them. When the insulation was damaged, the timing window widened or collapsed, and the thalamus could no longer reliably detect when two streams of activity should be treated as a single event.
In practical terms, that means the brain’s ability to match what you expect to see or hear with what actually arrives from the senses can break down, even if only a subset of fibers lose their myelin. The researchers showed that disrupting myelin in these Layer 5 pathways scrambled the coordination of activity between the cortex and thalamus, which are essential for attention, perception, and working memory. Their findings, published in Nature Comm, help explain why even subtle myelin damage can feel like a global cognitive slowdown.
How tiny gaps in insulation scramble sensory signals
The same principle appears in the way the brain handles sensory information. New research in mice shows that even a small loss of myelin around neurons in sensory pathways can severely disrupt how signals are encoded and relayed. Instead of crisp, well timed spikes that represent touch, sound, or vision, the affected neurons fire in a more erratic pattern, making it harder for downstream circuits to decode what is happening in the outside world.
In one study, scientists examined how demyelination in thalamic circuits altered the flow of sensory information and found that the timing and reliability of spikes were dramatically reduced, even when only a fraction of fibers were affected. The work revealed that the thalamus, which depends on precise timing to filter and prioritize inputs, became less effective at distinguishing signal from noise. This breakdown in encoding was highlighted in a report on how New research linked small losses of myelin to scrambled sensory signals, reinforcing the idea that the brain’s timing system is exquisitely sensitive to even localized damage.
From mouse circuits to human problems with memory and focus
What makes these findings so striking is how closely the mouse deficits resemble the earliest complaints people voice in clinics. When myelin is lost in just a few strategic spots, the brain’s internal timing drifts, and tasks that once felt automatic suddenly require more effort. Remembering a colleague’s name, following a fast moving group chat, or staying focused during a long Zoom meeting can all become harder, not because the information is missing, but because the signals that carry it are out of sync.
The mouse work on tiny patches of myelin loss explicitly connects these circuit level changes to everyday cognitive problems, noting that disruptions in corticothalamic timing can manifest as difficulty recalling familiar names or keeping up with complex thoughts. A broader overview of this research, framed as a health focused explanation of why losing a small amount of brain insulation can derail thinking, emphasizes that the damage does not need to be widespread to have real world consequences. That perspective is captured in a piece titled Scientists Discover Why Losing, which underscores how vulnerable complex cognition is to small, targeted losses of myelin.
Myelin loss, ageing, and the long arc toward neurodegeneration
These circuit level insights fit into a larger story about how the brain ages and why some people develop neurodegenerative diseases. Earlier work on white matter showed that a progressive loss of myelin is one of the defining features of the ageing brain, and that this erosion is closely tied to declines in processing speed and executive function. As the insulation thins and the brain’s ability to replace it falters, the timing of communication between distant regions becomes less reliable, setting the stage for more serious pathology.
Researchers studying age related brain deterioration have argued that myelin loss is not just a side effect but a driver of conditions like Multiple Sclerosis and other neurodegenerative disorders. In one report, scientists described how the brain’s wiring insulation is a major factor in age related decline and highlighted the limited capacity to regenerate that insulation once it is lost, a theme echoed in work on the loss of brain’s wiring insulation. When I connect those findings to the new mouse data, the picture that emerges is of a brain where small, early hits to myelin can gradually accumulate, tipping circuits from subtle timing glitches into full blown neurodegeneration.
CEMIP and the molecular triggers of myelin damage
Understanding that tiny patches of myelin loss can be devastating raises an urgent question: what starts the damage in the first place. One emerging answer centers on CEMIP, an enzyme that appears to be elevated in brain lesions linked to neurodegenerative conditions. Researchers have found that CEMIP is associated with areas of myelin damage and may play a direct role in breaking down the extracellular environment that supports healthy insulation.
In new work on this pathway, investigators reported that CEMIP levels are increased in brain tissue from people with disorders that involve demyelination and neurodegeneration. They argue that targeting this enzyme could help slow or prevent the cascade of events that leads from subtle myelin loss to widespread circuit failure. A detailed account of this research explains how CEMIP is linked to myelin damage and neurodegenerative conditions, and why it is now seen as a promising therapeutic target.
New compounds that aim to repair damaged insulation
If small gaps in myelin can derail thinking, then repairing those gaps becomes a powerful therapeutic goal. Researchers are now testing compounds that do more than just calm inflammation, instead trying to coax the brain into rebuilding its lost insulation. Two such candidates, K102 and K110, have drawn attention for their potential to repair nerve damage in Multiple Sclerosis by promoting remyelination rather than simply slowing further loss.
In one study, Researchers identified K102 and K110 as compounds that could restore myelin in animal models of Multiple Sclerosis and move the field closer to true regenerative therapy. The work suggests that these agents might help re wrap exposed nerve fibers, improving conduction and restoring more normal timing in affected circuits. A report on these efforts describes how Researchers see K102 and K110 as a bridge from basic research to potential clinical therapy, especially for patients whose cognitive symptoms stem from patchy myelin loss rather than complete nerve destruction.
Multiple Sclerosis as a proving ground for myelin repair
Multiple Sclerosis has long been the clearest example of what happens when myelin is stripped away, and it is now becoming a test bed for strategies that could also help people with subtler forms of cognitive decline. Traditional MS treatments focus on the immune system, trying to prevent new attacks on myelin, but they do little to rebuild what has already been lost. The new wave of therapies aims to change that by directly stimulating myelin repair and protecting vulnerable circuits.
One analysis of these efforts highlights how a compound called K102 may redefine the treatment of Multiple Sclerosis by promoting remyelination and improving nerve conduction. The report, titled Myelin Repair and Multiple Sclerosis, explains how K102 could help restore the brain’s wiring insulation and potentially reverse some of the cognitive and physical deficits that come from demyelination. If these approaches succeed in MS, they could open the door to similar strategies for age related myelin loss and other neurodegenerative conditions where tiny patches of damage add up over time.
Editing molecules and rethinking what is “reversible”
Alongside efforts to rebuild myelin, scientists are also probing whether targeted molecular edits can restore function in ageing brains. In one striking example, researchers showed that editing a single brain molecule could reverse memory loss in older animals, pointing to a new path for tackling cognitive decline. Instead of trying to fix every damaged cell, they focused on a key regulator of synaptic plasticity and demonstrated that precise intervention at that point could rejuvenate memory circuits.
The work suggests that some of the changes we associate with ageing may be more malleable than they appear, especially when combined with strategies that protect or repair myelin. A report on this approach describes how scientists were able to reverse memory loss by editing a single molecule, offering a new path to tackling memory loss in neurodegenerative diseases and age related cognitive decline. When I put that alongside the myelin work, the message is clear: even when tiny patches of insulation have already gone missing, the brain may still have room to recover if we can restore both its wiring and its molecular machinery.
Why tiny structural changes deserve big clinical attention
For clinicians and patients, the emerging science of myelin timing carries a practical warning. Waiting until brain scans show large, obvious lesions may mean missing the window when small, targeted interventions could make the biggest difference. The mouse data on corticothalamic fibers, the evidence that CEMIP is elevated in myelin damaged lesions, and the early promise of compounds like K102 and K110 all point to the same conclusion: microscopic changes can have macroscopic effects on how we think and feel.
At the same time, these findings offer a more hopeful narrative about cognitive decline. Instead of treating slower thinking or mild memory lapses as an inevitable slide, they invite us to see them as potential signs of specific, treatable breakdowns in the brain’s insulation and timing systems. As studies on Study implicating enzymes like CEMIP, work by Larry Sherman, Ph.D., and advances in myelin repair and molecular editing converge, I see a future in which protecting and restoring tiny patches of brain insulation becomes a central strategy for preserving thought itself.
More from MorningOverview