
Researchers at the University of California, San Diego, have developed cerium oxide nanoparticles that effectively reduce inflammation and fibrosis in models of alcohol-related liver disease. These nanoparticles target oxidative stress and immune responses, as demonstrated in preclinical studies showing up to a 50% reduction in liver damage markers. This innovative approach builds on previous research, highlighting the potential of nanoparticles to modulate hepatic stellate cells and prevent scar tissue formation in ethanol-induced liver injury models.
Understanding Alcohol-Related Liver Disease
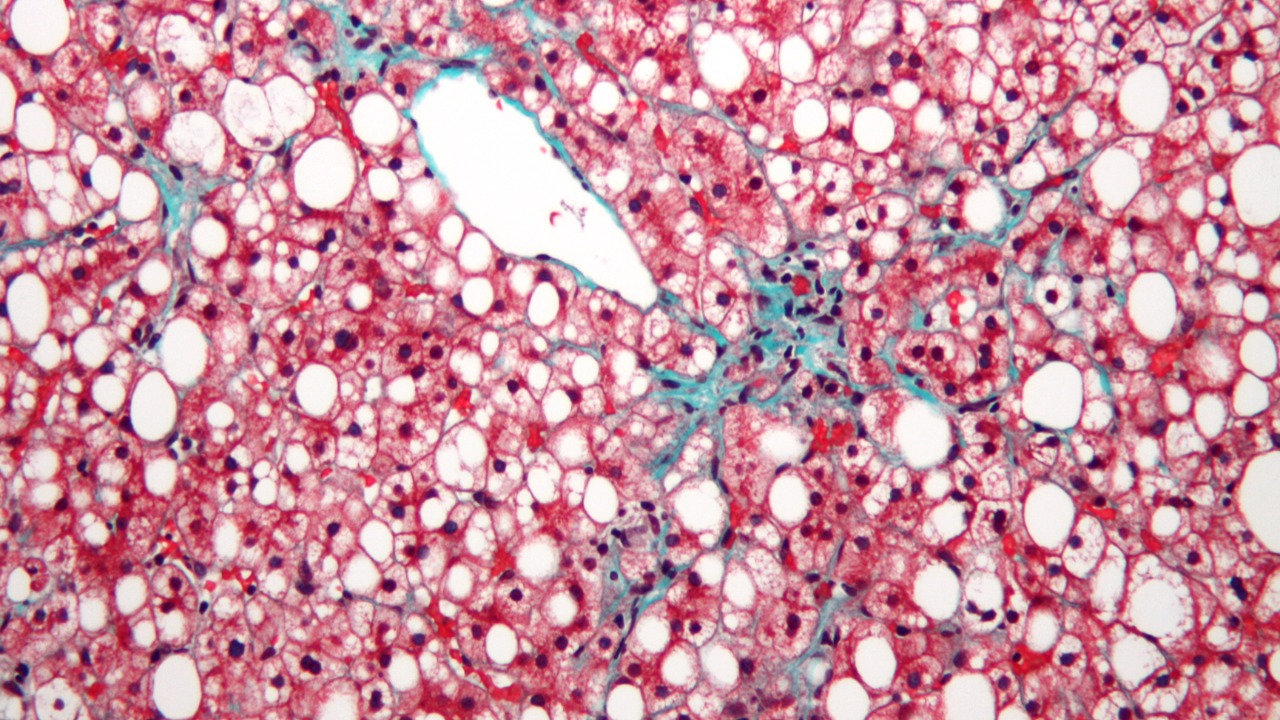
Alcohol-related liver disease (ALD) is a significant health concern, affecting over 10 million people annually in the United States. Chronic alcohol consumption can lead to a progression from steatosis to inflammation and eventually cirrhosis. This progression is largely driven by the harmful byproducts generated during alcohol metabolism, which contribute to oxidative stress and activate fibrogenic pathways. The role of oxidative stress and reactive oxygen species (ROS) in the pathogenesis of ALD is well-documented, with these byproducts causing damage to hepatocytes and promoting liver fibrosis.
Despite the prevalence of ALD, treatment options remain limited. Currently, abstinence from alcohol is the only proven intervention, and there are no FDA-approved therapies specifically targeting advanced fibrosis in ALD patients. This lack of effective treatments underscores the urgent need for new therapeutic strategies that can address the underlying mechanisms of liver damage and fibrosis in ALD.
Mechanism of Nanoparticles in Liver Protection
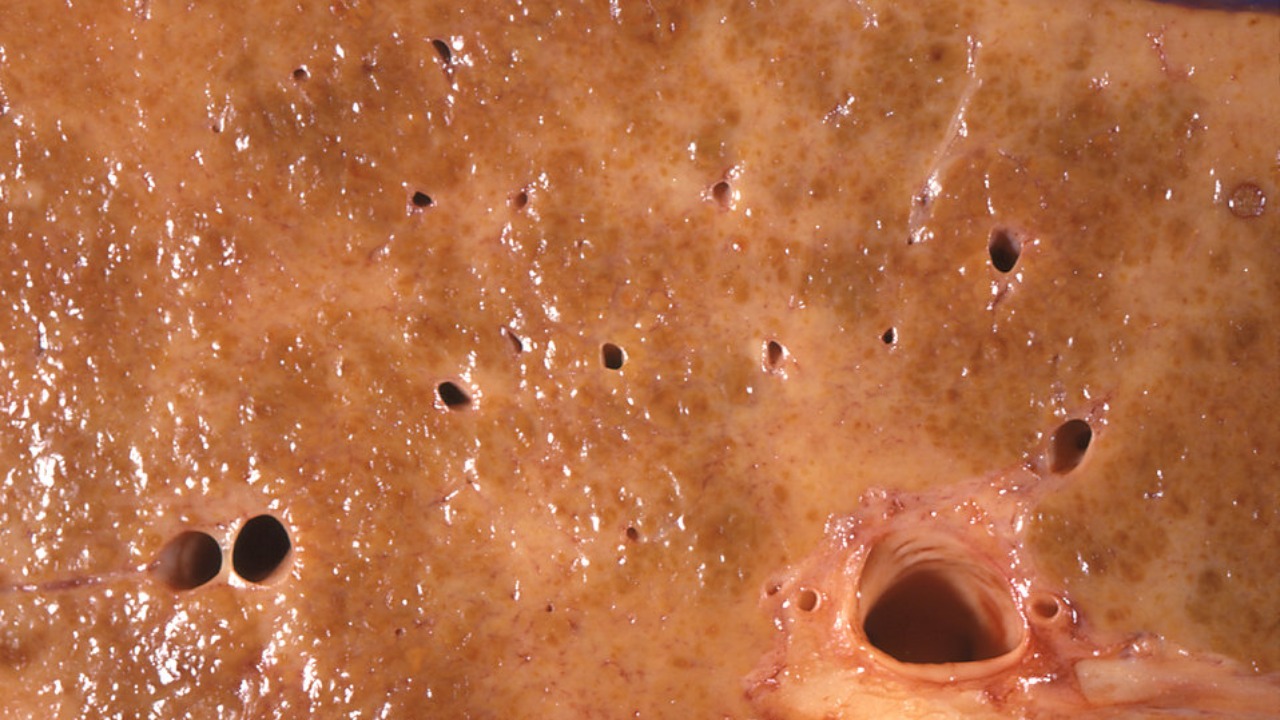
Cerium oxide nanoparticles offer a promising approach to liver protection by acting as potent antioxidants. These nanoparticles can scavenge reactive oxygen species and mimic the activity of superoxide dismutase, thereby preventing lipid peroxidation in liver cells exposed to ethanol. This antioxidant capability is crucial in mitigating the oxidative stress that contributes to liver damage in ALD.
The targeted delivery of nanoparticles to inflamed liver tissues is achieved through surface modifications, which enhance their uptake by Kupffer cells and reduce systemic toxicity. This targeted approach ensures that the therapeutic effects of the nanoparticles are concentrated in the liver, minimizing potential side effects in other organs. In vitro experiments have demonstrated that nanoparticle treatment can inhibit the expression of pro-inflammatory cytokines like TNF-α and IL-6 by up to 40% in ethanol-challenged macrophages, highlighting their potential to modulate immune responses in ALD.
Preclinical Evidence and Study Results
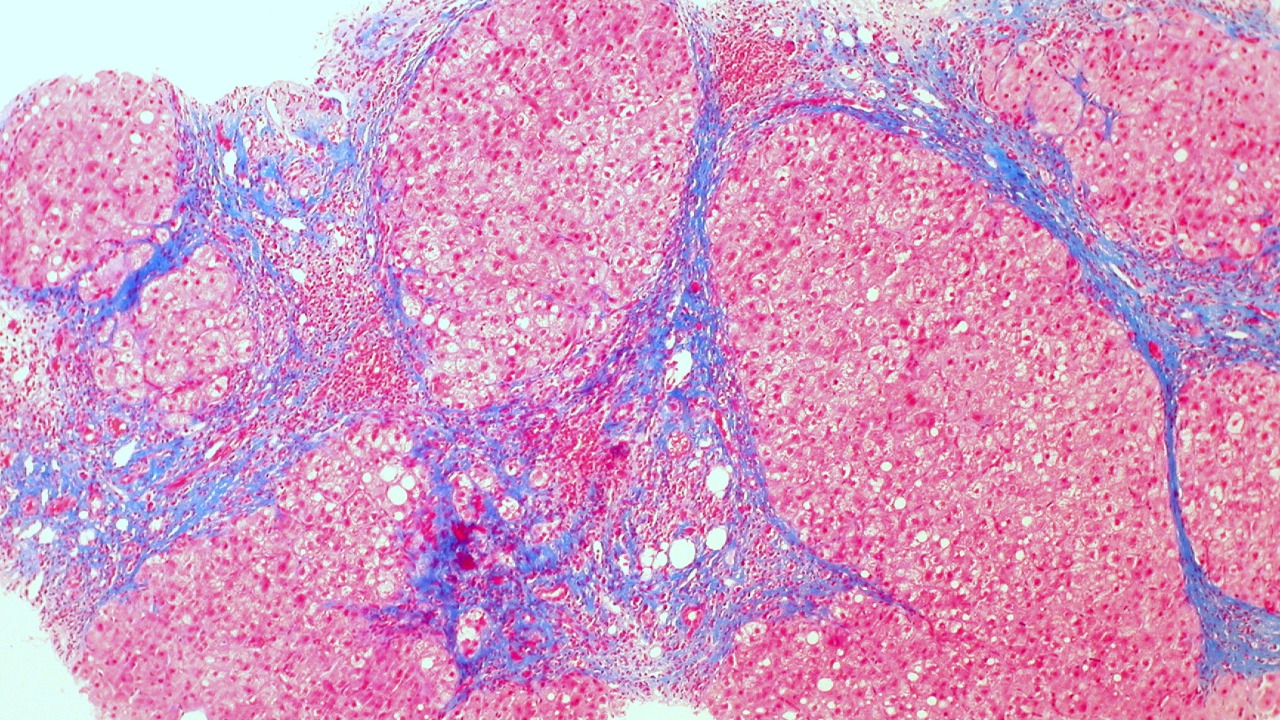
Preclinical studies using mouse models of chronic alcohol feeding have shown promising results for cerium oxide nanoparticles. When administered intravenously at a dose of 0.5 mg/kg, these nanoparticles significantly preserved liver architecture and reduced collagen deposition by 60% after eight weeks. This reduction in collagen deposition is a critical indicator of decreased fibrosis, suggesting that nanoparticles can effectively prevent the progression of liver damage in ALD.
Histological data from these studies indicate a decrease in the activation of hepatic stellate cells, which are key drivers of fibrosis. Nanoparticle-treated livers exhibited lower expression of α-SMA, a marker of stellate cell activation, compared to controls. Additionally, biochemical markers such as ALT and AST levels were lowered by 30-50% in treated groups, alongside improved glutathione levels, demonstrating the hepatoprotective effects of the nanoparticles.
Challenges in Translating to Clinical Use
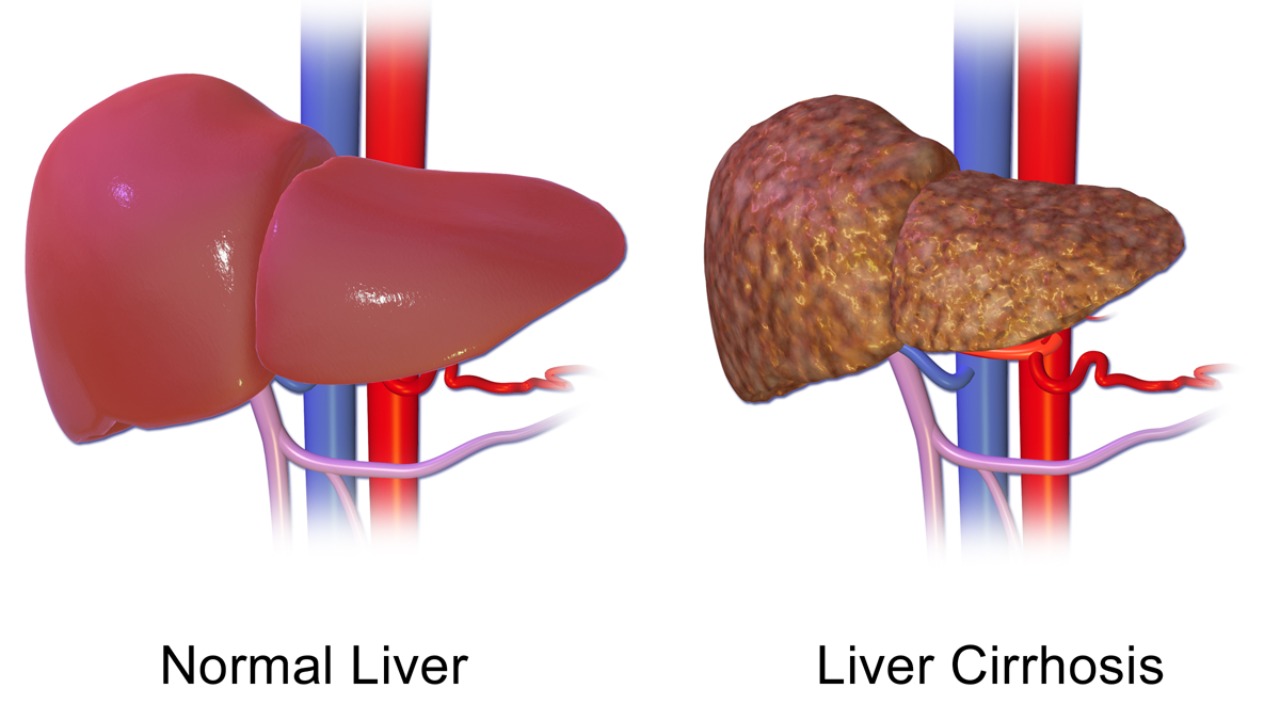
Despite the promising preclinical results, several challenges remain in translating nanoparticle therapies to clinical use. One major concern is the potential for biocompatibility issues, including the accumulation of nanoparticles in non-target organs like the spleen. Long-term rodent studies have highlighted the need for further optimization to ensure human safety. Addressing these biocompatibility concerns is crucial for the successful development of nanoparticle-based therapies for ALD.
Additionally, scalability and regulatory hurdles must be overcome before these therapies can be widely adopted. While preclinical efficacy is promising, Phase I clinical trials are necessary to evaluate the appropriate dosing and safety of nanoparticles in ALD patients with varying disease stages. These trials will be essential in determining the feasibility of nanoparticle therapies for human use.
Future Directions for Nanoparticle Therapies
Looking ahead, researchers are exploring combination strategies to enhance the efficacy of nanoparticle therapies. Pairing nanoparticles with anti-fibrotic drugs like obeticholic acid could offer synergistic effects, improving outcomes in patients with advanced ALD. Preliminary studies have shown promising results for such combination therapies, suggesting a potential path forward for more effective treatment options.
Ongoing research is also focused on developing personalized nanoparticle formulations tailored to individual genetic variants in alcohol metabolism enzymes, such as ADH1B. By accounting for genetic differences, these personalized therapies could improve efficacy across diverse populations, offering a more targeted approach to treating ALD. As research progresses, these advancements hold the potential to transform the landscape of ALD treatment, providing new hope for patients affected by this debilitating disease.
For more detailed information, you can read the full study on Phys.org and explore related research in Frontiers in Pharmacology.